Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Physical Chemistry
Unique Multicenter Bond Confirmed
Spectroscopic evidence provides ultimate proof that a two-electron bond between four carbon atoms in a radical dimer really exists
by Stephen K. Ritter
May 6, 2013
| A version of this story appeared in
Volume 91, Issue 18
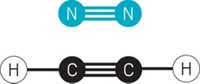
Chemical bonds typically involve two atoms sharing one or more pairs of electrons. But chemists keep finding molecules that defy that norm. For example, some electron-deficient molecules exhibit multicenter bonding with three atoms sharing two electrons. A dozen years ago, Joel S. Miller of the University of Utah and his colleagues discovered an unusual type of multicenter bonding: a molecule with a four-atom, two-electron bond. Miller’s team came across the anomaly when preparing magnetic materials from transition-metal complexes that include the radical anion tetracyanoethylene (TCNE). In the crystal structures they found that two TCNE units form a dimer (shown) in which the central carbons are separated by 2.9 Å. That distance exceeds conventional C–C bond lengths, which are in the range of 1.5 Å, but it is still close enough to suggest a bonding interaction. In addition, the dimer’s structural, spectroscopic, and magnetic properties point to a four-carbon, two-electron bond that is stronger than hydrogen bonds. “But there has still been a concern whether the bond really exists,” Miller says. His team now has definitive proof after elucidating telltale vibrational flexing of the bond by Raman spectroscopy (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201207813). Better understanding of this unique bonding could ultimately lead to new molecules with interesting magnetic and other properties, Miller notes.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter