Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy Storage
New Lithium-Ion Battery Woes
Safety: Airplane battery fires thrust electrochemistry safety back in the spotlight
by Mitch Jacoby
January 25, 2013
| A version of this story appeared in
Volume 91, Issue 4

Questions about the safety of using lithium-ion batteries for transportation applications have hit a zenith with two incidents in which such battery units onboard Boeing 787 Dreamliner airplanes caught fire or began to smolder. Airlines worldwide have grounded the new wide-body jets pending the outcome of investigations concerning the batteries and supporting equipment.
Boeing says the batteries on its 787s feature multiple backups to ensure safety, including protections against overcharging and overdischarging. It has declined to comment on the investigations.
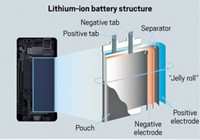
Li-ion batteries can pack more energy into smaller and lighter weight units than other types of batteries. Those attributes have spurred enormous growth in their use for cell phones, laptop computers, and other portable electronic devices. Boeing selected the low-weight, high-energy-density batteries for the 787 Dreamliner to help reduce the new jetliner’s overall weight and bulk and thereby increase fuel efficiency.
A downside of Li-ion cells, however, is that they contain a flammable electrolyte solution consisting of lithium salts in organic solvents such as ethylene carbonate and ethyl methyl carbonate. This is not the case for other commercial battery types.

In Li-ion cells, heat generated by an internal or external short circuit, abusive electrical conditions, or other sources can, under some circumstances, ignite the battery liquid or rapidly raise its vapor pressure until the cell bursts, says Daniel H. Doughty of Battery Safety Consulting in Albuquerque, N.M.
Reports of fires in portable electronics caused by Li-ion batteries led manufacturers to recall millions of laptop batteries several years ago. The news shoved Li-ion battery safety issues into the spotlight. The recent incidents with the 63-lb battery units onboard Boeing jets have grabbed headlines in part because of the obvious difference from laptop computers in scale and potential danger.
Investigators have not yet determined the cause of the Boeing battery pack incidents that occurred earlier this month. In one case, a Japan Airlines (JAL) 787 Li-ion battery that powers the plane’s auxiliary power unit caught fire on Jan. 7 as the empty plane sat on the tarmac at Boston’s Logan International Airport. In the other incident, All Nippon Airways (ANA) pilots made an emergency landing in central Japan on Jan. 16 because of alarms warning of an electrical problem and an unusual odor in the 787’s cockpit. Both the alarm and the odor were traced to a damaged Li-ion battery.
The U.S. National Transportation Safety Board has ruled out excess voltage as the cause of the JAL fire. The agency says examination of flight recorder data indicates the auxiliary power unit battery did not exceed 32 V, the designed operating voltage.
Likewise, Norihiro Goto, Japan’s Transport Safety Board chairman, says flight recorder data show that the ANA battery’s output voltage was normal before alarms sounded in the cockpit.
Brian Barnett, a battery safety specialist at Lexington, Mass.-based technology development firm Tiax, says safety risks can be minimized with electronics to monitor battery performance.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter