Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Metal Duo Makes β-Aryl Ketones
Palladium and silver forge a ketone motif that is common in drugs and agrochemicals
by Carmen Drahl
December 2, 2013
| A version of this story appeared in
Volume 91, Issue 48
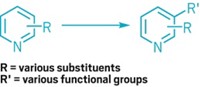
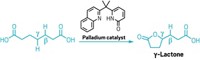
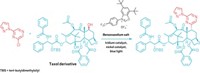
The carbons immediately adjacent to a carbonyl group are usually straightforward places to form new carbon-carbon bonds. Just use a strong base, or a carefully designed catalyst, and that α-carbon is good to go. The next carbon over, the β-carbon, is not so amenable to change, however. Traditionally, β-substituted carbonyls require multiple steps to prepare. Because that β-motif is common in insecticides and drug candidates, chemists are keen to simplify the process. They’ve developed a handful of β-functionalization reactions, but none is generally applicable yet. Now, graduate student Zhongxing Huang and assistant professor Guangbin Dong of the University of Texas, Austin, have added to the toolbox with a reaction that inserts aromatic rings at the β-position of simple ketones (J. Am. Chem. Soc. 2013, DOI: 10.1021/ja410389a). The palladium-catalyzed process uses readily available aryl iodides and bromides as the substrates. A silver salt regenerates the active catalyst. Dong cautions that the reaction is still in its infancy. He’d like to figure out the reaction mechanism and replace the silver reagent with something less expensive. UT Austin is pursuing a provisional patent on the chemistry.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter