Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Toxicology
Caffeine Jitters
Sales boost in energy drinks and deaths linked to the products make scientists and regulators worry about safe levels of the stimulant
by Lauren K. Wolf
February 4, 2013
| A version of this story appeared in
Volume 91, Issue 5

When Matthew Penbross woke on a morning in August 2007—a day in which he’d be competing in motocross races near Port Macquarie, Australia—he wanted to be prepared. To improve his reaction speed on the start line, the 28-year-old began drinking Red Bull Energy Drinks soon after he rolled out of bed. All told, he would drink seven or eight of the caffeinated beverages within a seven-hour period.
After his second race that day, Penbross wasn’t feeling well. But despite some short-lived chest pains, he kept riding and went on to win a race in the afternoon. Twenty minutes after he crossed the last finish line of the day, though, his heart stopped.
Penbross had no family history of heart disease, and he regularly drank a few Red Bulls per day, news reports later stated. The hospital doctors who treated his collapse and helped him recover went on to suggest that the excessive amount of caffeinated energy drinks he consumed was the likely culprit (Med. J. Aust.2009,190, 41).
Stories like Penbross’ have surfaced with increasing frequency in recent years. According to a report released last month by the U.S. Substance Abuse & Mental Health Services Administration, the annual number of emergency room visits associated with energy drinks increased to 20,000 in 2011, a 36% boost from the previous year. Late last year, the New York Times reported that the U.S. Food & Drug Administration is investigating reports of five deaths linked to Monster Energy drinks and 13 deaths linked to 5-Hour Energy shots.
To download a PDF of this article, visit http://cenm.ag/caffeine (C&EN, February 4, 2013, pages 9–12).
To download a PDF of this article, visit http://cenm.ag/caffeine (C&EN, February 4, 2013, pages 9–12).
As concerns about the safety of these products have risen, so too have their sales. According to SymphonyIRI Group, a Chicago-based market research firm, the market for caffeinated energy drinks hit $9.8 billion last year.
“People often don’t understand the potential risk of these beverages,” says Bruce A. Goldberger, director of forensic toxicology at the University of Florida’s pathology labs. Caffeine is a stimulant and, when consumed at high enough levels, can have negative effects.
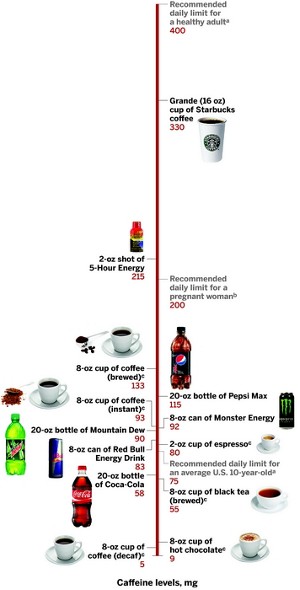
Monster Energy vigorously defends its safety record. In response to the fatality reports FDA is investigating, the company released the following statement: “Neither the science nor the facts support the allegations that have been made. Monster reiterates that its products are and have always been safe.” The firm also says a 16-oz can of its product contains only about half the caffeine found in a 16-oz cup of premium coffee, such as Starbucks.
Caffeine safety has proven hard to measure. Although scientists have established the toxic dose to be somewhere around 10 g, they say that the value can fluctuate depending on how a person processes the stimulant. Caffeine gets cleared from the body at different rates because of genetic variations, gender, and even whether a person is a smoker. For this reason, it’s difficult to set a safe limit of daily consumption on the compound. Physiological differences, as well as differences in the way people consume caffeine, have tied FDA in knots as it has debated how to regulate the substance.
Caffeine has long been prized for its ability to increase a person’s alertness and energy. According to lore, these properties were noted in the 9th century by an Ethiopian goatherd who found his flock frolicking after eating coffee berries from nearby bushes. What is not lore is that caffeine is one of the most frequently ingested pharmacological substances in the world. People proclaim their love of the chemical by displaying its structure on T-shirts, mugs, and jewelry. According to FDA, at least 80% of adults in the U.S. consume caffeine every day.
Scientists know a lot about how the popular stimulant triggers alertness in the body at low doses, says Bertil B. Fredholm, emeritus professor of pharmacology at the Karolinska Institute, in Sweden. Once the compound gets absorbed into the bloodstream, it moves to the liver, where it is metabolized. There, cytochrome P450 enzymes yank different methyl groups off caffeine to transform it into the primary metabolites paraxanthine, theophylline, and theobromine.
Caffeine and its metabolites subsequently bind to proteins called adenosine receptors located throughout the body. When they bind to two of these receptors—named A1 and A2A—the stimulants block the proteins from interacting with their natural partner, the small molecule adenosine. Normally, adenosine’s interaction with its receptors regulates nerve cell activity as well as the release of neurotransmitters such as dopamine. It also promotes sleepiness.
But when caffeine or its metabolites prevent adenosine from doing its job, dopamine and other neurotransmitter levels increase, leading to a surge in nerve activity in the brain and on the heart. This action then causes a jump in heart rate and blood pressure.
Like other indulgences in life, however, too much of a good thing can be bad. “Whereas low-dose caffeine effects are wakefulness, a little bit of arousal, and slight euphoria,” Fredholm says, “high-dose effects are anxiety, irritation, and general mental discomfort—a completely different kettle of fish.” Those negative high-dose effects are especially worrisome, given that researchers still don’t fully understand their origins.
“High-dose caffeine effects are much more complex,” Fredholm explains. “It’s still unknown precisely what the primary mechanism of action is in the brain and elsewhere.”

The toxic level in humans, about 10 g, is roughly the equivalent of imbibing 75 cups of brewed coffee (in 8-oz mugs) or 120 cans of Red Bull over a few hours.
But that lethal limit can vary widely from person to person, experts say. Caffeine is processed differently in each individual, so setting a precise safety limit is not a straightforward matter.
And standard animal models haven’t been much help. “Mice and rats—the animals we’d normally use to determine lethal doses of substances—metabolize caffeine much more quickly than humans,” says James D. Lane, a medical psychologist at Duke University Medical Center. So it’s hard to compare the health effects of caffeine in rodents and humans, he adds.
For these reasons, scientists have struggled to distinguish benign, low doses of caffeine from troublesome, high doses.
In 2003, a team at Health Canada, a government regulatory agency, reviewed more than 200 studies about caffeine’s effects on human health. On the basis of the survey, the team concluded that 400 mg of caffeine per day (or about three 8-oz cups of brewed coffee) is a safe dose for healthy adults to consume (Food Addit. Contam., DOI: 10.1080/0265203021000007840). At and below this level, the average person does not experience negative mood changes or heart problems, the report stated.
The Health Canada team also recommended a limit of 2.5 mg per kg body weight per day for children. For an average 10-year-old in the U.S., that’s about 75 mg of caffeine, or two 12-oz cans of Coca-Cola.
But these recommended “safe” levels of caffeine are just statistical averages over the population. Some people tolerate caffeine and can ingest large quantities of the compound without ill effects, and others can’t, explains Emma Childs, a behavioral psychopharmacologist at the University of Chicago.
Fifty percent of the caffeine a person takes in gets cleared from the body, on average, in five hours. But studies have shown this rate can vary because of other drug use: Women taking oral contraceptives break down caffeine slower than those not on a contraceptive pill, and people who smoke process the stimulant faster than those who don’t.
Genetics also plays a big role in how a person reacts to caffeine. For example, men metabolize caffeine faster than women.
Scientists have also found that certain DNA sequence variations in the gene coding for CYP1A2, the main cytochrome P450 enzyme that metabolizes caffeine, control how fast or slowly a person breaks down the stimulant. The variations, called single-nucleotide polymorphisms (SNPs), increase the levels of the enzyme present in the liver.
Also influencing how people react to caffeine are SNPs in a gene called ADORA2A, which codes across the body for the adenosine receptor A2A. For instance, Childs and her group showed in 2008 that study participants with a particular SNP in ADORA2A began feeling anxious after consuming only 150 mg of caffeine. Participants without the SNP didn’t experience caffeine-induced anxiety until they had ingested 450 mg of the substance (Neuropsychopharmacology, DOI: 10.1038/npp.2008.17).
Fortunately, Childs says, “people with the ADORA2A polymorphism are pretty cognizant of it.” The gene defect is usually associated with panic disorder, she adds. “So those people know how much they’re allowed to have and when they’ve had too much.”
It’s more difficult, Childs says, to advise slow caffeine metabolizers who have polymorphisms in their CYP1A2 liver enzyme. “Without genetic testing,” she explains, “it’s hard to know whether you need to be careful with intake.”
Because of the variability in caffeine sensitivity among people, FDA has stopped short of recommending a consumption limit for the entire population. The agency goes only so far as to state on its website, “Experts agree that 600 mg (four to seven cups of coffee) of caffeine or more each day is too much.”
The agency has also found it difficult to regulate the caffeine content of food and drink. Caffeine is a psychoactive drug, but it is also a natural ingredient in widely consumed beverages such as coffee and tea.
At one point in the early 1980s, FDA considered prohibiting the addition of synthetic caffeine to soft drinks for safety reasons. But beverage manufacturers claimed the compound was a necessary flavor enhancer for their products.
The agency relented, but it put a limit of 0.02% on the amount of compound allowed to be added to cola-type beverages. That’s a concentration of about 6 mg per oz, a threshold that drinks like Pepsi Max (5.8 mg per oz) bump up against but don’t surpass.
But “beverage” limitations don’t apply to drinks like Monster Energy or 5-Hour Energy. These caffeine-rich products are sold as something different: dietary supplements. The 1994 Dietary Supplement Health & Education Act allows drinks marketed this way to contain ingredients such as vitamins, minerals, herbs, and other substances that supplement a person’s diet. And if one of those substances is caffeine, it is free of FDA’s 0.02% food-additive restriction.
As a result, drinks such as Monster Energy may contain caffeine levels in excess of 6 mg per oz. And because the beverage manufacturers sometimes include caffeine as part of a drink’s proprietary “energy blend,” the amount of the stimulant doesn’t need to be reported on the bottles. These blends include compounds such as glucuronolactone and taurine, although experts agree that caffeine is the active ingredient delivering the power boost.

FDA doesn’t have enough evidence to stop the practice, says Daniel Fabricant, director of FDA’s Division of Dietary Supplement Programs. “For FDA to seek a limit on any ingredient in a dietary supplement, we have to demonstrate that there is a significant or reasonable risk of illness or injury under the normal conditions of use or in the labeling,” Fabricant says.
That happened for Canada in 2011 when it announced a limit of 180 mg of caffeine per energy-drink serving. A cap hasn’t been instituted in the U.S., but FDA says it is taking seriously reports of five deaths and about 30 adverse events possibly linked to Monster Energy that have occurred since 2008.
FDA released those records after a Freedom of Information Act request was submitted by Wendy Crossland, the mother of a teenage girl who died in 2011 of a heart arrhythmia. The 14-year-old, Anais Fournier, consumed a 24-oz Monster Energy drink at a mall near Hagerstown, Md. The next day, she drank another 24-oz can and then collapsed.
Each can of Monster contains an estimated 240 to 280 mg of caffeine, a daily dose that is considered safe for adults. Fournier, however, was younger and smaller than the average adult and had a preexisting heart condition.
Advertisement
“Caffeine will increase adrenaline in your body, and it will increase blood pressure,” says Duke’s Lane. For habitual users, “it generally increases the wear and tear on your body.” Healthy adults don’t typically notice these mild effects, but the changes can exacerbate underlying conditions such as heart disorders, particularly at acute doses.
Reports of adverse events connected with 5-Hour Energy shots are also being scrutinized by FDA. Since 2009, the agency has received about 90 filings related to the product, including records of 13 deaths. Unlike energy drinks, which sit on grocery store shelves next to sodas and come in 8- to 24-oz cans, energy shots are sold next to the Tic Tacs and gum at the cash register in 2-oz servings.
Even though 5-Hour Energy bottles do not list caffeine content, in December, Consumer Reports said the shots contain 215 mg of the stimulant. That’s almost seven times the concentration of an average cup of brewed coffee and 19 times the 0.02% FDA limit for beverages.
Living Essentials, the maker of 5-Hour Energy, has responded to the scrutiny by issuing commercials about its product safety and by claiming each shot contains the amount of caffeine in a cup of premium coffee, such as Starbucks.
“But energy shots aren’t cups of coffee. It’s not comparing apples to apples,” says Goldberger, the University of Florida toxicologist. “Your typical cup of coffee comes hot; you wouldn’t usually gulp it down.” On the other hand, he says, “energy shots come in 2-oz containers that can be gulped.”
Lack of data is something else FDA has been grappling with, says supplements director Fabricant. “Looking into adverse event reports is challenging because quite often, you don’t have a lot of background information,” such as the person’s medical history, age, and size or details about exactly what happened. “But we’re always reviewing and rereviewing the reports,” he adds.
The number of drinks it took to cause an adverse event isn’t listed in the records submitted to FDA either. But a survey of calls to an Australian poison information center between 2004 and 2010 suggests that number could be a factor in why people get sick. About 200 calls related to energy drinks came in during the seven-year period, and the average subject consumed five beverages before feeling ill (Med. J. Aust., DOI: 10.5694/mja11.10838).
“It’s generally recognized that caffeine is a mildly addictive stimulant drug,” says Michael F. Jacobson, executive director of the Center for Science in the Public Interest (CSPI), a consumer health advocacy group. Some young adults view it as a legal way of getting high.
The average subject involved in the Australian poison center calls, for instance, was 17 years old. And 30% of the time, the subject used the energy drinks in combination with alcohol or drugs like Ecstasy.
“The Federal Trade Commission probably needs to look into the marketing of these products,” Goldberger contends.
That’s because the amped-up message attached to energy drinks is quite different from the relaxed, good-to-the-last-drop one associates with coffee, he says. One energy drink, called Cocaine, once labeled itself “the legal alternative.”
It’s not just beverages that have been caught up in the caffeine craze: Ballpark favorite Cracker Jack will soon come as Cracker Jack’d—2-oz packages of the familiar candy-coated kernels jammed, in certain flavors, with 70 mg of caffeine from coffee. According to the label, they’re “snacks with impact.”
Cracker Jack’d will also join caffeine-laden candies, waffles, and even pancake syrup. So far, these items are sold mostly on the Internet and haven’t caused problems, says CSPI’s Jacobson, “but if they prove to be popular, you can bet there’s going to be an escalation of small companies selling them in stores. Major marketers might then see the new niche and capture their share of the profits.”
Frito-Lay, the maker of Cracker Jack’d, has responded to concerns about its product line by saying it stands behind the safety of its caffeine-containing kernels. “All marketing for the products will be exclusively aimed at adult consumers, and the package design and appearance are wholly different from Cracker Jack to ensure there is no confusion among consumers,” Frito-Lay says.
CSPI’s Jacobson is not convinced. The trend of pumping products full of caffeine, he argues, deserves attention from FDA before it gets out of hand.
To download a PDF of this article, visit http://cenm.ag/caffeine (C&EN, February 4, 2013, pages 9–12).




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter