Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Business
Europe Invests $550 Million In Drug Discovery And Antibiotic Development
Pharmaceuticals: EU effort aims to revitalize drug discovery and development in Europe
by Alex Scott
February 14, 2013
| A version of this story appeared in
Volume 91, Issue 7

The European Union’s Innovative Medicines Initiative (IMI) has unveiled new programs to develop antibiotics and accelerate early drug discovery. The separate programs, which aim to revitalize drug design and development in Europe, feature public and private funding totaling more than $550 million.
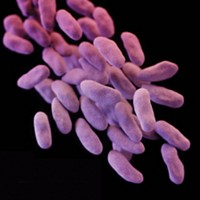
IMI’s New Drugs for Bad Bugs, a joint program between industry, academia, and biotech organizations, will combat antibiotic resistance in the EU by promoting collaboration within the region’s antibiotic development community. The program will receive $300 million in funding from the European Commission and drug firms.
The lion’s share of the funds will go to Combacte, a project to create a clinical research system on antibacterial resistance. The program’s second project, Translocation, will investigate new pathways for getting antibiotics into bacteria.
“These new projects will give a fresh impetus to the search for new weapons to fight the drug-resistant pathogens that have already killed so many in Europe and elsewhere,” says Michel Goldman, IMI’s executive director.
A separate initiative, called the European Lead Factory, aims to accelerate early drug discovery and provide therapies for unmet medical needs. The EC and drug companies will provide the Lead Factory with more than $250 million in funding over five years. A consortium of 30 partners from academia, small firms, nonprofits, and drug companies will participate.
A key component of the Lead Factory is an effort to collect and screen more than 500,000 small molecules against potential biological targets. Seven drug companies will contribute more than 300,000 compounds; academia and smaller companies will provide the rest. Drug companies and a newly established European Screening Centre at sites in Newhouse, Scotland, and Oss, the Netherlands, will carry out the screening.
The efforts are ambitious, but not everyone is convinced they will have a major impact. Industry consultant Girish Malhotra, who counts pharmaceutical firms among his clients, points out that the investments are tiny compared with what a single big pharmaceutical company puts into drug discovery. The program does “not have enough money to identify and do any preliminary testing,” he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter