Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Water
Oil Dispersants Used During Gulf Spill Degrade Slowly In Cold Water
Oil Spill Response: Persistence of Corexit 9500 at deep-sea and Arctic temperatures suggests more research is needed on the chemicals’ toxicity, researchers say
by Mark Schrope
February 13, 2013

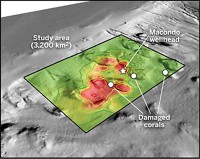
During the 2010 Deepwater Horizon oil spill in the Gulf of Mexico, clean up crews applied millions of liters of oil dispersants both at the ocean surface and in the deep sea. At the time, the public and some scientists worried about the environmental effects of the chemicals, in particular how long they would last in the deep sea. According to a new Environmental Protection Agency study, the key active ingredient in the dispersants degrades very rapidly under conditions similar to those found at the Gulf surface during the spill. Meanwhile, in the much colder temperatures found in the deep sea, the breakdown is quite slow (Environ. Sci. Technol., DOI: 10.1021/es303881h).
Dispersants break up oil into tiny droplets so bacteria and chemical processes can degrade it more quickly. To try to lessen the harm caused by the oil released into the Gulf, oil spill responders initiated what would turn out to be the second largest application of dispersants in history. Also, for the first time, the crews pumped dispersants 5,000 feet below the ocean surface—into the Deepwater Horizon’s blown wellhead to break up oil as it gushed into the Gulf.
EPA oil spill expert Albert D. Venosa, working with academic researchers, wanted to better understand the degradation of Corexit 9500, the main dispersant used during the spill. They ran tests on artificial seawater at 5 ºC, about the temperature at the wellhead, and at 25 ºC, roughly the temperature of the surface waters during the hot summer of the spill.
One key round of experiments involved adding an oil and dispersant mix to flasks of water that the scientists had inoculated with bacterial communities. For the cold water flasks, the team used bacteria isolated from the deep Gulf, and for the warm water, they used microbes from shallow water.
Using liquid chromatography tandem mass spectrometry, the team tracked levels of dioctyl sodium sulfosuccinate (DOSS), Corexit 9500’s main surfactant. In the warm water, when mixed with oil, DOSS broke down quickly, with most of the compound gone in eight days.
In the cold water tests, however, the team didn’t observe significant dispersant breakdown for almost a month, and some DOSS persisted at the end of the 42-day experiment. The reason, the authors say, is likely that at low temperatures the microbes slowly produce the enzymes needed to chew up DOSS.
The results fit well with previous observations in the Gulf: Months after the spill ended, teams still detected DOSS at very low concentrations in the deep sea (Environ. Sci. Technol., DOI: 10.1021/es103838p).
In the paper, the authors conclude that their results on DOSS’ persistence at cold temperatures suggest more research is needed to understand the chemical’s toxicity and that of its breakdown products in colder environments. Cold water conditions are a concern not only for deep sea oil spills, they write, but also for spills in Arctic waters.
David L. Valentine, a microbiologist at the University of California, Santa Barbara, was part of the group that measured deep-sea DOSS levels during the spill. He says the EPA work gives researchers an improved understanding of dispersant fate in different environments and the mechanisms driving the breakdown of the compounds. However, he suggests caution when applying the results directly to actual spills because other key factors, such as levels of nutrients bacteria need, may differ from the laboratory conditions.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter