Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Using Force To Pattern Organic Compounds On Graphene
Materials Science: Polymer tips push cyclopentadiene-containing inks into graphene, creating covalent linkages between the ink and the two-dimensional material
by Prachi Patel
June 25, 2013

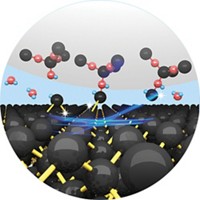
To fabricate electronic devices that take advantage of graphene’s conductivity and strength, materials scientists first need to chemically modify the material to tweak its other properties. Attaching biomolecules, for instance, could enable new types of sensors, and patterning graphene with impurities would make the material behave like a semiconductor, needed for many electronic devices.

Unfortunately, graphene often resists such functionalization. Now chemists report that a little force does the trick (J. Am. Chem. Soc. 2013, DOI: 10.1021/ja4042077).
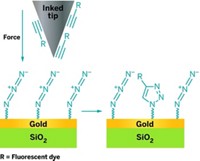
A team led by Adam B. Braunschweig at the University of Miami and Kendall N. Houk at the University of California, Los Angeles, attached molecules in patterns on graphene’s surface via Diels-Alder reactions accelerated by pressure. Other researchers had shown that graphene can participate in Diels-Alder reactions either as the diene or the dienophile, the two types of reactants in the ring-forming reaction (J. Am. Chem. Soc. 2011, DOI: 10.1021/ja200118b). It’s also well known that pressure accelerates the Diels-Alder reaction.
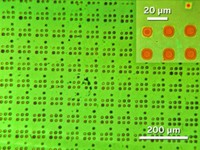
So the chemists coated an array of 80-nm-wide polymer tips with inks containing various molecules linked to cyclopentadiene, a classic Diels-Alder reactant. For one of the inks, they attached the dye cyanine3 to cyclopentadiene. The researchers mounted the tip array onto an atomic force microscope and gently pushed the tips into the surface of graphene. The cyclopentadienes reacted with the graphene, resulting in 20 µm by 40 µm patches of graphene decorated with patterns of dye dots.
The technique works at ambient temperatures and pressures, the chemists note. They are now trying to use the method to functionalize graphene with carbohydrates to make biological sensors.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter