Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Nanoparticles Could Disrupt Immune Cell Function
Nanotoxicology: After treatment with iron oxide particles, macrophages fail to ingest pathogenic bacteria, one of the cells’ key jobs
by Erika Gebel
July 17, 2013

A new study suggests there’s more to nanoparticle toxicology than cell life and death. Although immune cells treated with iron oxide particles appeared healthy in standard toxicology tests, they struggled to perform one of their key jobs: engulfing pathogenic bacteria (ACS Nano 2013, DOI: 10.1021/nn402145t). The researchers wonder if exposure to significant levels of the nanoparticles could lead to dysfunction in people’s immune systems.
As nanoparticles continue to appear in consumer products, toxicologists want to understand how exposure to the materials affects human health. So far, many studies have involved exposing human or animal cells to engineered nanoparticles and monitoring cell survival. But these tests may not tell the whole story, says Brian D. Thrall of the Pacific Northwest National Laboratory. “Much of the work has looked at direct acute effects—cell deaths or inflammation—of nanoparticles,” Thrall says. “If you only look at direct acute effects, you may be missing changes in cell function that may be important.”
For example, he says, scientists have tested silica and superparamagnetic iron oxide nanoparticles on cells and concluded that they have fairly low toxicity. Yet, in previous work, he found that silica nanoparticles can alter the gene expression of macrophages, immune cells that help clear foreign particles and pathogenic bacteria from the body (PLoS One 2011, DOI: 10.1371/journal.pone.0014673). Such cellular changes could indicate that, even though the cells appear healthy, their ability to respond to pathogens has been impaired. To test this, Thrall and his colleagues exposed macrophages to these particles and then checked to see if the cells could still clear out bacteria.
The researchers grew a culture of mouse bone-marrow macrophages and added 25 µg/mL of silica or iron oxide particles. That probably represents a high dose for people, Thrall says, but it may be relevant for those who work around the nanoparticles. In general, researchers have little data on actual nanoparticle exposure levels for people, he says.
Next, the scientists added a lipopolysaccharide to the culture, which the macrophages treat as foreign bacteria. The team monitored the cells’ responses by measuring gene expression with a microarray chip device.
In macrophages treated with silica particles, 44 genes that are normally regulated by lipopolysaccharides had expression levels that were 50% greater or lower than those levels in nanoparticle-free cells. Meanwhile, treatment with iron oxide nanoparticles altered the expression levels of 1,044 genes by 50%. The most troubling changes, Thrall says, took place in oxidative stress and inflammation pathways, which play a significant role in how the macrophages detect and clear bacteria. “This suggested that the normal innate immune function is significantly altered,” Thrall says.
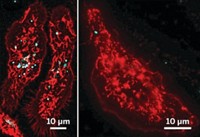
To see if the changes in gene expression impaired the cells’ ability to deal with pathogens, the researchers mixed the macrophages with Streptococcus pneumonia, the bacteria that cause pneumonia. Untreated macrophages and those exposed to the silica nanoparticles ingested the bacteria with gusto, as measured by flow cytometry. However, the cells exposed to iron oxide nanoparticles struggled to take up the bacteria: They ingested about half the number of pathogens relative to the untreated cells.
Robert L. Tanguay of Oregon State University says these results suggest that nanoparticles may need additional safety testing on top of the standard toxicity studies. “That could open up a huge Pandora’s box,” he says, adding to the number of variables researchers consider when assessing nanomaterial toxicity.
But he points out that this study only examined individual cells. “These results would need to be challenged in a whole animal,” Tanguay says, to determine if the macrophage changes translate into impaired health. Thrall is currently testing whether animals exposed to nanoparticles are more likely to develop lung infections.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter