Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Prion Protein Fragment Hints At Infection Mechanism
Structural Biology: A small piece of the human prion protein forms oligomers with tails that could interact with healthy prions and convert them to infectious forms
by Sarah Webb
July 16, 2013

Prion proteins sit in cell membranes throughout the body, but they have one troubling characteristic: When they misfold, they can become infectious, recruiting other prions to change shape. Those misfolded proteins wreak havoc in brain tissue, leading to neurological diseases. Now researchers studying the crystal structure of a snippet of the human prion have found clues to how oligomers of the proteins could coax others to misfold (J. Am. Chem. Soc. 2013, DOI: 10.1021/ja403001q).
Prion researchers have long sought a precise, atomic-resolution structure of the infectious form of the prion protein, says Witold K. Surewicz of Case Western Reserve University. Understanding the structural changes that prion proteins undergo could help scientists figure out not only how the proteins cause others to misfold, but also how prions in one animal species can cause infections in other species, such as between cows and humans in mad cow disease.
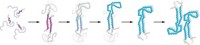
But detailed structural data has been limited because the twisted, large amyloid fibrils formed by misfolded prions are not amenable to crystallization, preventing researchers from studying them using X-ray crystallography, Surewicz says. Instead, researchers have studied small peptide segments of the larger protein that can form microcrystals. Structural biologists use structures of these fragments to learn more about the larger structures.
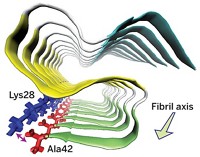
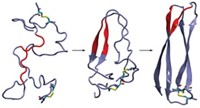
In the current study, Surewicz and postdoc Marcin I. Apostol decided to examine a 12-amino-acid portion of the human prion protein containing a disulfide bond. When they looked at these peptides’ structures, they found something surprising: Instead of forming twisting structures that look like amyloid fibrils, the peptides formed groups of hexameric oligomers rich with amino acids arranged in pleated β-sheets. It was an interesting finding, Surewicz says, because prion researchers have been debating whether the fibril structure or another oligomeric protein structure is the toxic prion species.
Each oligomer has a tail consisting of amino acids in a β-strand that can form hydrogen bonds with neighboring peptides. The researchers suggest that these tails could explain how oligomer structures bind to and potentially perturb the structure of other peptides. Still, Surewicz cautions that it’s always dangerous to try to interpret the behavior of full proteins from the structures of parts.
It’s an “intriguing and novel structure,” says Simon Sharpe of The Hospital for Sick Children in Toronto. Though the structure provides important insights into possible features of amyloid oligomers, he says, it’s unlikely that this particular structure will reflect the exact structure of full prion oligomers in a living organism.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter