Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Cross-Coupling Yields Tertiary Fluorides
A new catalytic enantioselective Negishi cross-coupling process delivers a diverse array of tertiary alkyl fluorides
by Stephen K. Ritter
April 14, 2014
| A version of this story appeared in
Volume 92, Issue 15
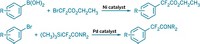
By using an asymmetric Negishi cross-coupling strategy, Yufan Liang and Gregory C. Fu of California Institute of Technology have developed a catalytic enantioselective synthesis of acyclic tertiary alkyl fluorides (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja501815p). Motivated by applications in biomedical research and materials science, chemists have been on a tear in recent years creating strategies to increase the diversity of organofluorine compounds. In the case of alkyl fluorides, several research groups have made advances in preparing α-fluoroaldehydes in which a secondary stereogenic carbon-bearing fluorine sits next to a carbonyl group. The Caltech team’s new method uses a nickel bis(oxazoline) catalyst to couple α-bromo-α-fluoroketones with arylzinc reagents to form α-fluoroketones containing a tertiary fluorine-substituted carbon (shown). The researchers varied both the aryl and alkyl groups on the starting fluoroketone and the aryl group on the zinc reagent to come up with an array of tertiary alkyl fluorides in moderate yields and high enantiomeric excesses. Liang and Fu show that the α-fluoroketones can be further derivatized to other families of organofluorine molecules by additions to the carbonyl group and by selective Baeyer-Villiger oxidations to form fluoroesters.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter