Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Colloids Yield Full Color Palette
Core-shell nanoparticles within microcapsules create novel pigments that get their color from a physical phenomenon
by Bethany Halford
April 28, 2014
| A version of this story appeared in
Volume 92, Issue 17

Vinothan N. Manoharan holds up a small vial of liquid. It looks something like milk—white and opaque and otherwise unremarkable. But the particles that make up this suspension could one day be used to create paints and inks that won’t fade or full-color displays that don’t need to be backlit. The key, says Manoharan, a professor in Harvard University’s physics department and School of Engineering & Applied Sciences, is using some clever chemistry to bring about a physical phenomenon known as structural color.
Much of what we see gets its color from colored molecules. There are, for example, aniline dyes, such as mauve, and metal compounds, such as cobalt blue, that give color to objects. There’s a relationship between these molecules’ electronic structures and how they absorb and reemit certain wavelengths of light.
Structural color has nothing to do with a molecule’s electrons. Instead, structural color arises on the supramolecular level when a material’s nanostructures selectively reflect certain wavelengths of light while letting all other wavelengths pass through.
“With structural colors you can make different colors from exactly the same material,” explains Sofia Magkiriadou, a graduate student in Manoharan’s lab. Because structural colors aren’t based on molecules, which can react and change color, they tend to be more resistant to fading and bleaching.
During Magkiriadou’s first week working with Manoharan, he asked her to take the white liquid and remove water from the colloidal suspension. When she did, the material turned blue, a result of the colloidal particles packing together in such a way that they reflected wavelengths of blue light.
This came as no surprise to Manoharan. Other researchers had made so-called photonic pigments, or pigments based on structural color, by making arrays of nanoparticles. Ordered nanoparticle arrays give rise to iridescent pigments, which physicists describe as anisotropic. Disordered arrays, physicists had recently learned, would also produce colored pigments. But these have a matte appearance, which physicists describe as isotropic. For paint or display applications, it’s the matte appearance that everyone is after.
Manoharan was pleased to see that the colloids Magkiriadou had dehydrated took on a blue matte appearance. But other researchers had already made isotropic blue and green photonic pigments. Manoharan and his students had bigger plans: They were hoping to use the same colloids to make a rainbow of pigments through structural color—a feat no one had accomplished yet.
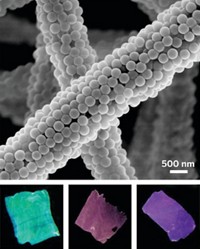
To understand the Manoharan lab’s system, one must envision spheres within spheres within spheres. The smallest, innermost spheres are polystyrene nanoparticles. These make up the core of the core-shell nanoparticle colloids, and they are what physically scatter the light in Manoharan’s photonic pigments.

The polystyrene nanoparticles are surrounded by a shell of poly(N-isopropylacrylamide-co-acrylic acid) or poly(NiPAm-AAc). This shell is transparent, so light can pass through it. It is also soft and can squish as the core-shell particles pack together. Modifying the distance between the polystyrene cores, by varying the shell thickness, makes them reflect different wavelengths of light, thereby producing different colors.
Jin-Gyu Park, a postdoc in the Manoharan lab, and Shin-Hyun Kim, currently a professor at Korea Advanced Institute of Science & Technology, came up with a way to use microfluidics to encapsulate an aqueous suspension of the core-shell nanoparticles within an ultraviolet-curable monomer, ethoxylated trimethylolpropane triacrylate. “That monomer shell is permeable to water, so you can shrink it down, and as you shrink it down you compress those particles,” Manoharan explains. “As you suck more water out, you actually change the distance between the particles. So you can tune in color that way.” The final step is to polymerize the monomer and lock the capsule, nanoparticles, and color into place.
Using this system, Manoharan’s team was able to produce structural color across the full visible spectrum (Angew. Chem. Int. Ed. 2014, DOI: 10.1002/anie.201309306). That’s impressive, says Eric Dufresne, a physicist at Yale who studies structural color and soft materials. “In our lab when we tried to make deep greens and reds with structural color it just didn’t work,” he says. “The Manoharan lab has taken a different architecture and designed and synthesized new colloidal materials that allow you to make red. I think that’s potentially going to be very exciting.”
Getting the red photonic pigment was probably the biggest hurdle, Manoharan says. “These things were inspired by bluebirds. Those birds use structural color to get that blue,” he says. Red birds, however, don’t use structural color. Instead they get their hue from colored molecules in their feathers. It occurred to Manoharan and his team that because nature doesn’t make isotropic red with structural color it simply might not be possible for them to do it. But this work, Manoharan says, shows that it is possible to make isotropic structural color in red, albeit a red that the researchers would like to look more saturated.
If you can make red, green, and blue, you can make all the colors you need for a color display that doesn’t need to be backlit and therefore has a longer battery life. That’s just one of the applications Manoharan has in mind for these photonic pigments. He says they could also be used in fade-resistant paints and inks, although he’s quick to add they haven’t yet shown that the pigments can go for years without losing their color.
“What we have now is a proof of concept,” Manoharan says. “There’s still some work that needs to be done before we can put these into applications.”
COLOR THEORY
Structural Color 101
Bluebirds, butterflies, and berries of Pollia condensata all get their vivid blue hues from a phenomenon known as structural color. Structural color comes about on a supramolecular level when a material’s nanostructures scatter light in such a way that certain wavelengths get reflected and amplified through constructive interference. This is different from dyes, which achieve color from their molecules’ electrons. In fact, the molecules within structurally colored objects are usually colorless. Grind up these materials and that nanostructure is lost along with the color.
The opal is a classic example of an object that achieves remarkable colors via structural interference. Opals have silica spheres, about 200 nm in diameter, that are arranged in a highly ordered fashion. The silica is inherently colorless, but interference from the light that scatters through different parts of that ordered silica structure gives rise to an opal’s color.

Physicists describe opals, peacock feathers, Morpho butterflies, and even compact discs as examples of anisotropic structural color. That is, their color changes with viewing angle. There is also isotropic structural color, in which the color doesn’t change with viewing angle. The blue feathers of the eastern bluebird, for example, get their vivid hue from isotropic structural color. Isotropic structural color also arises from nanoscale structures, but they must be disordered to keep the color independent of the viewing angle.
Objects with structural color tend to resist fading. A couple of years ago, researchers at England’s Royal Botanic Gardens discovered that the blue color of P. condensata fruit comes from the nanostructure of its cellulose fibers, accounting for the fact that the fruit is as bright today as the day it was picked 40 years ago. This suggests that man-made structural color could also be fade-resistant, making such materials attractive for paint and ink applications.







Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter