Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Complex Carbohydrates
Improvements in methods, standards, and software are still needed to move mass spec analysis of carbohydrates into the mainstream
by Celia Henry Arnaud
June 9, 2014
| A version of this story appeared in
Volume 92, Issue 23

Carbohydrates are ubiquitous. They densely carpet cell surfaces. They are the main component of the extracellular matrix that surrounds cells. They are involved in many aspects of cell-cell communication.
Glycans, a type of carbohydrate, are “the first thing a signaling molecule encounters when it contacts a cell,” says Robert J. Linhardt, a biochemist at Rensselaer Polytechnic Institute who studies carbohydrate structure and function. “They’re the first thing a virus encounters when it infects a cell.” As many as 80% of human proteins are thought to be decorated with carbohydrates, he notes.
Despite the importance of carbohydrates, characterization methods for them have lagged behind those for their fellow biopolymers, nucleic acids, and proteins. In mass spectrometric analyses of glycosylated proteins, scientists often strip the carbohydrates away and focus on the protein. Even when they try to zero in on the carbohydrates, researchers often must be satisfied with incomplete information that tells them composition but not how those carbohydrates are put together.
Researchers are coming to realize that they can no longer ignore carbohydrates. And if they want to determine the relationship between structure and function, they need more than a cartoon understanding of carbohydrate structure.
Mass spectrometry—alone and in combination with liquid chromatography—is making headway in providing that detailed understanding. Improved analytical methods are providing information on carbohydrate structure, including pinpointing isomers and defining linkages between sugars. But two hurdles stand in the way of their broader adoption: the lack of standards that can make such analyses truly quantitative and the lack of software that can make data analysis and interpretation accessible to nonexperts.
Many of the challenges in analyzing carbohydrates stem from how the biopolymers are made. Nucleic acids and proteins are encoded by templates. That means that the universe of possible sequences is already known and circumscribed. As a result, the sequence of a gene reveals the sequence of the protein it encodes. Carbohydrates have no such template.
Carbohydrate synthesis starts in the endoplasmic reticulum, a cellular structure studded with ribosomes where proteins are made. Branched chains of sugars are assembled on lipid-linked precursors and then transferred to protein nitrogen atoms during translation. These N-linked glycoproteins are transferred to another organelle, the Golgi, where some sugars are trimmed away and many others are added. Also in the Golgi, other complex carbohydrates are attached to protein oxygen atoms.
“What happens in the endoplasmic reticulum is universal. All cells have the same machinery,” says Yehia Mechref, an analytical chemistry professor at Texas Tech University who studies carbohydrates. “What happens in the Golgi is cell-specific.” Each cell has its own set of Golgi enzymes that trim and customize the sugars.
“The Golgi is the least-understood organelle,” Linhardt says. “We don’t know the control points in the Golgi that actually determine carbohydrate structure. We do know that it’s responsive to the environment and the organism’s needs at the time.”
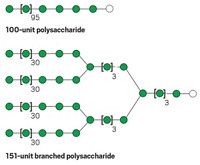
The resulting carbohydrates are a complex mixture. By some estimates, these variations add up to thousands of possible glycans. They can contain a range of monosaccharides, many of which are isomers of one another. They can have different branching patterns and different linkages between the sugars. They can be linked to proteins in different ways. These subtle structural differences can have profound effects on glycan function. And these subtle differences make carbohydrates a challenge to analyze by mass spectrometry.
But the situation might not be so dire, according to Ron Orlando, a mass spectrometrist at the Complex Carbohydrate Research Center at the University of Georgia. Carbohydrates may not have a template, but they are made via a defined biosynthetic pathway.
“The biosynthetic pathway doesn’t allow for every possible combination to be produced,” Orlando says. “If you’re looking at human glycans, there are only certain building blocks available.” That limits the possible glycan masses that could be encountered.
Carlito B. Lebrilla, a mass spectrometrist at the University of California, Davis, agrees. From a chemical perspective, there are often many more combinatorial possibilities than biology actually uses. “If you look at it from the chemical point of view, you could make all these different combinations, but I think biology is simpler than that,” he says.

To illustrate he points to immunoglobulin G, a type of heavily glycosylated antibody. It has approximately 70 different glycan compositions. Each of those compositions might exist as about five isomers for a total of about 350 types. The problem is not identifying an unlimited number of compositions and isomers, he notes—it’s determining which glycans are attached to particular sites.
Vernon N. Reinhold, head of the Glycomics Center at the University of New Hampshire, advocates multistage tandem mass spectrometry, so-called MSn, as the key to determining carbohydrate sequence and structure. He has spent more than a decade developing and trying to convince other mass spectrometrists of the benefits of his approach.
The more common approach of doing MS/MS is “totally inadequate for detailing glycans,” even when coupled with LC, Reinhold asserts. “You need to look at every linkage and every branch, as well as consider the stereochemistry,” he says.
Reinhold believes that “high resolution is of little value when trying to understand the structural isomers of linkage and branching.” Lower resolution ion trap mass analyzers are readily available, cost-effective, and extremely powerful for unraveling structural detail, he says.
To analyze glycans, Reinhold blocks all free hydroxyls with methyl groups, infuses the protected glycans into the instrument by electrospray, and selectively isolates each glycan for activation and disassembly. Reinhold’s team has developed a library of the resulting fragments. Although multiple disassembly stages are possible, it’s rare that they need to go further than MS5, he says.
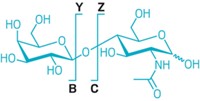
Reinhold’s method works for the N- and O-linked glycans of proteins and lipids and the free glycans of milk. It doesn’t work so well for ionic glycans such as heavily sulfated glycosaminoglycans (GAGs). GAGs, such as the anticoagulant heparin, are linear polysaccharides made of repeating disaccharide units. The disaccharides consist of an amino sugar and a uronic sugar.
Sulfation makes GAGs chemically challenging to deal with in a mass spectrometer, says Joseph Zaia, a professor of biochemistry and associate director of the Center for Biomedical Mass Spectrometry at Boston University School of Medicine. “Sulfates are very acidic. Inside a mass spectrometer they can be labile unless you take a lot of care. We’re still not able to precisely measure the mass of intact GAGs.”
But some headway is being made. I. Jonathan Amster, a mass spectrometrist at the University of Georgia, has teamed up with Rensselaer’s Linhardt to develop methods for sequencing GAGs.
“GAGs have some regularity to their structure,” Amster says. “It’s the modifications that are really challenging. Most methods developed for protein analysis can’t be directly adapted for getting at sites of sulfation.”
Amster and Linhardt successfully sequenced the GAG portion of the simplest proteoglycan, a molecule called bikunin, which is found in plasma and serves as a proteinase inhibitor. The math suggested that there were hundreds of millions of possibilities for bikunin sulfation.
They were surprised at what they found: Bikunin’s GAGs are far more regular than they anticipated.
Amster attributes their success in sequencing GAGs to choosing the right fragmentation method. “We used some of our more complicated electron-based methods for sequencing, but they weren’t working,” Amster says. “We tried the simple peptide method of collisional dissociation. That cracked these glycans apart.”
Improvements in fragmentation methods are also helping scientists sequence other glycans. Especially important are methods that result in so-called cross-ring cleavages—cleavages within a particular sugar ring—instead of just cleavages between rings.
“Cleavage between rings will tell you how residues are lined up with respect to one another on a branching structure, but you don’t know the position of the linkages unless you get fragmentation within the sugar rings themselves,” says Catherine E. Costello, professor of biochemistry and director of Boston University’s Center for Biomedical Mass Spectrometry. For generating cross-ring cleavages, electron-based dissociation methods are particularly effective and provide extensive linkage information in a single step, she says.
Zaia is particularly optimistic about a specific activation method. “Electron transfer dissociation in the negative mode is particularly useful for glycosaminoglycans,” he says. “But not all commercially available instruments will do electron dissociation in the negative mode.”
Although mass spectrometry of glycans continues to improve, many researchers prefer to combine mass spec with separation methods. “I would rather separate and simplify my mass spec challenge than ignore separations and just make it a really complicated mass spec problem,” Georgia’s Orlando says.
Pauline M. Rudd, head of glycoscience research at the National Institute for Bioprocessing Research & Training, in Dublin, Ireland, has been a pioneer in coupling chromatography with mass spec for glycan analysis. She combines enzyme digestion with chromatography and mass spectrometry. The enzymes, which remove terminal sugars, are monosaccharide- and linkage-specific. The enzymes plus the chromatographic retention times are enough to work out the structure of most glycans, Rudd says. She then uses mass spec as a confirmatory method.
Texas Tech’s Mechref is likewise developing methods to define glycan structures on the basis of their chromatographic retention times and their mass-to-charge ratios. His goal is to develop glycomics methods that use the same setup as a proteomics analysis. Lack of quantitative standards that can be used to define retention times is slowing the process down.
“People use glycans derived from glycoproteins rather than using glycan standards,” he says. “Synthesis of such standards is not trivial. When you talk to people doing carbohydrate synthesis, they always say, ‘Yes, we could do it.’ But when you ask about the time frame, they say it will take years.”
Standards that could be used to optimize instrument conditions for glycan analysis could make such analyses more popular. “The tuning conditions under which most instruments are set up are optimized for peptide and protein analysis,” Costello says.
Standard mixtures could help researchers determine the best parameters for glycan analysis on particular instruments. Researchers “can learn to set these things up and get good glycan data, which would be better than they would get if they ran it under standard peptide analysis conditions,” she says.
But perhaps the biggest hurdle to more widespread adoption of mass-spec-based glycomic analysis is the challenge of data analysis and interpretation. Most mass spec software was written for handling peptides and proteins, Costello says. “It needs modifications to make it user-friendly for carbohydrates.”
For instance, for selecting masses for tandem mass spectrometry, instrument software is typically programmed to look for predicted masses of peptides. The software may not use the best mass windows to select peaks for glycans. “Some companies are appreciating the needs of glycobiology and are working on and starting to market software that is more adapted to glycan analysis,” Costello says. The lack of good data interpretation tools means that analyses take much longer than desired.
Zaia’s group has written software to interpret glycan mass spectra. “We wrote our software GlycReSoft to assign glycan compositions and abundances,” he says. The first version of the software was developed for LC/MS profiling of GAGs. It incorporates masses, retention times, and abundances. A second version of the software incorporates MS/MS mass spectra and is being expanded to other types of glycans.
Improved data analysis methods could lead to much-needed glycan spectral databases. “Although there have been years of activity, we do not have an accurate database that we can take MS/MS data and just search against,” Mechref says. Proteomics researchers have long benefited from databases of protein sequences.
Existing databases of carbohydrate sequences are not nearly as useful, he says. One of the problems is that a particular glycan can be represented differently in different databases. “There’s work to get to a single nomenclature that everyone would use,” Costello says. In the meantime, there’s a need to translate the existing nomenclatures from one database to another.
Glycomics experts hope that continued improvements will make the technology accessible to more scientists. “We’d like to develop technologies that would be applicable by anyone at a university with a big core facility,” Linhardt says. “You’ve got to push the boundaries of what instruments can do, but at the same time, you’ve got to make it accessible to the biologists and chemists who want to do this work.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter