Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Spying On Crystal Formation
Microscopy: Study reveals complex, multipath nature of nucleation and growth
by Mitch Jacoby
September 8, 2014
| A version of this story appeared in
Volume 92, Issue 36
Crystal nucleation and transformation events often happen suddenly and spontaneously, leaving researchers with little chance to study these microscopic processes in detail. But under the right conditions and discerning eye of a powerful probe, these everyday events can reveal their secrets.
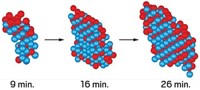
For a team of scientists using a customized electron microscope, conditions were just right to watch crystals of calcium carbonate nucleate, grow, and undergo change (Science 2014, DOI: 10.1126/science.1254051).
The study shows that traditional theories indicating that crystals form via nucleation followed by orderly growth are incomplete. It also shows that one of the most abundant materials on Earth forms via multiple, often simultaneous mineralization pathways.
“For a decade, we’ve been studying formation pathways of carbonates using high-powered microscopes, but we hadn’t had the tools to watch the crystals form in real time,” says team leader James J. De Yoreo of Pacific Northwest National Laboratory. Now, the team has such a tool—a microscope flow cell that enables scientists to image liquid-phase reactions at the nanoscale.

The group, which includes Michael H. Nielsen and Shaul Aloni of Lawrence Berkeley National Laboratory, loaded tiny quantities of solutions of sodium bicarbonate and calcium chloride into the cell. Under select flow-rate and concentration conditions, crystals suddenly began to grow and transform, revealing previously unseen events.
In some cases, amorphous dropletlike particles of CaCO3 formed in solution. Suddenly, crystals of the carbonate minerals aragonite and vaterite appeared on the surface of the particles and grew at the particles’ expense. In other cases, the group observed particles of calcite and other CaCO3 phases suddenly nucleating near one another in solution and growing independently.
“We are at an exciting moment in time in which the detailed in situ imaging of common liquid-phase reactions such as crystallization of calcium carbonate has come into reach,” says microscopist Nico A. J. M. Sommerdijk of Eindhoven University of Technology, in the Netherlands. He adds that the study confirms the existence of multiple and sometimes simultaneous crystallization pathways that some researchers suspected were present but could not verify on the basis of ex situ experiments.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter