Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Atomic Insights On Shark Teeth
Imaging method allows researchers to nondestructively view biomineralized material on the atomic scale
by Bethany Halford
January 27, 2014
| A version of this story appeared in
Volume 92, Issue 4
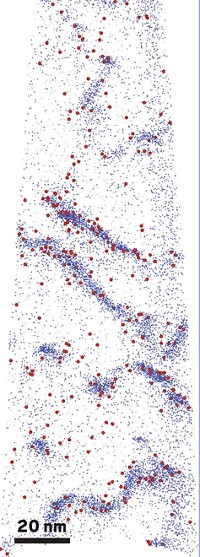
Considering that neither brushing nor flossing is part of a shark’s daily dental regimen, the animals get remarkably few cavities. To get an idea of what makes shark teeth so resistant to decay, researchers in Japan aimed a transmission electron microscope at the enamel on the creature’s chompers. Normally the microscope’s electron beam can damage biomineralized material. But by using low-dose imaging techniques, Yuichi Ikuhara and Zhongchang Wang of Tohoku University and colleagues were able to minimize such damage and directly image every individual atom in the enamel (Angew. Chem. Int. Ed. 2014, DOI:10.1002/anie.201307689). The enamel is made of fluorapatite, Ca5(PO4)3F, which appears to the researchers as hexagons of calcium, phosphorus, and oxygen atoms with fluorine atoms at their centers. Making calculations based on these images, they determined that fluorine is partially covalently bound to calcium in the enamel. This suggests that fluorine is critical to stabilizing the hexagonal frames. Loss of fluorine atoms would leave atom-sized holes and weaken the teeth.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter