Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Enzymes And Metals Enable Regiodivergent Organic Reactions
Judicious choice of engineered enzymes or transition-metal catalysts enables researchers to prepare different products from the same reaction
by Stephen K. Ritter
November 3, 2014
| A version of this story appeared in
Volume 92, Issue 44

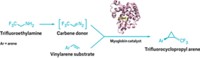
Divergent regioselectivity, in which the same reaction can lead to different products depending on the catalyst and reaction conditions, is a particular challenge for synthetic organic chemists. Two research teams have reported findings that provide new insight on how to make directional selectivity a little easier. Frances H. Arnold and coworkers at California Institute of Technology engineered two variants of a cytochrome P450BM3 enzyme to control nitrogen atom transfer and enable regiodivergent C–H aminations. One enzyme favors ring-closing amination at the α-position of an alkyl substituent on a benzene sulfonyl azide. The other enzyme favors amination at the β-position (J. Am. Chem. Soc. 2014, DOI: 10.1021/ja509308v). Meanwhile, Chao-Jun Li’s group at McGill University, in Montreal, has discovered a selectivity switch in ring-forming coupling reactions between 2-hydroxybenzaldehydes and terminal alkynes. With a gold catalyst, the researchers obtain an isoflavanone skeleton, whereas a rhodium catalyst produces a coumarin skeleton (Angew. Chem. Int. Ed. 2014, DOI: 10.1002/anie.201407589). According to the researchers, enzymes represent a versatile platform for solving selectivity problems in organic synthesis, either through synthetic biology or through inspiration to develop metal-catalyst enzyme mimics.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter