Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Electronic Materials
Tapping Solar Power With Perovskites
Low cost and impressive performance thrust new solar-cell technology into spotlight
by Mitch Jacoby
February 24, 2014
| A version of this story appeared in
Volume 92, Issue 8
Every now and again, a well-studied research topic explodes with new life. Long after carbon materials filled chapters of dated textbooks, for example, that field’s soul was reenergized around 1990 after buckyballs and carbon nanotubes were discovered. It happened again in that field about a half-dozen years ago when graphene took the world by storm. It’s happening now in photovoltaics.
“It seems we’ve all been bitten by the perovskite bug,” says University of Oxford physicist Henry J. Snaith, naming the topic in photovoltaics that’s all the rage these days. Snaith leads Oxford’s Photovoltaic & Optoelectronic Device Group.
Photovoltaic devices that directly convert sunlight to electricity aren’t new. Various types of these energy transformers, which are also known as solar cells, have been around for decades. The type Snaith refers to, perovskite solar cells, has been studied intensely only in the past year and a half. During that time, numerous researchers joined the effort, attracted by these cells’ promise to be inexpensive yet high performing. These scientists quickly boosted perovskite solar cells’ electrical output. Now they are working to broaden the cells’ appeal by tailoring their chemical compositions to further boost electrical output, by improving processing methods and stability, and by figuring out why these solar cells perform so unexpectedly well.

The stodgy, Cold War-era quality to the name “perovskite” belies the excitement engulfing this research topic. Strictly speaking, the label refers to a long-ago-discovered calcium-titanium-based mineral composed mainly of calcium titanate, CaTiO3. Scientists use the term loosely nowadays to refer to a large class of materials that, like CaTiO3, exhibit ABX3 stoichiometry and adopt the perovskite crystal structure.
The perovskites rocking the photovoltaics world these days are organometal trihalides, the most commonly studied of which is CH3NH3PbI3. (CH3NH3 is the A group in ABX3.) The main reason for the excitement is the recent steep rate of improvement in perovskite solar-cell performance.
Some types of solar cells, for example, ones based on high-purity crystalline silicon, have long provided top-notch photovoltaic performance. The power conversion efficiency, or simply the efficiency, of these devices, which is a ratio of the light energy in to the electrical energy out, reaches around 25%. But that performance is costly, owing to the expensive materials and energy-intensive crystal growth and vapor deposition methods needed to make such cells. State-of-the-art solar cells boasting efficiencies topping 40% are available today. But those specialized research devices come with much higher price tags.
Other technologies, such as organic photovoltaic devices based on photosensitive organic polymers, made waves when they debuted in the solar-cell arena because they hold the promise of being less expensive. These devices benefit from being made via low-cost solution-phase plastics manufacturing methods.
Although the price was right for plastic solar cells, the conversion efficiency in the early-2000s time frame was only a few percent. Since then, the value has climbed slowly and now sits around 11%. Other low-cost solar-cell technologies also improved slowly during that period. But they, too, remain relatively weak converters of solar energy.

In contrast, perovskite solar cells are following a more radical course. Almost overnight the conversion efficiency of these cells leaped from just a few percent in a forerunner to perovskite cells to more than 16% in current versions. Most of the advances were reported in 2012 and 2013. The fast-paced improvement, which hasn’t shown signs of slowing, coupled with inexpensive materials and preparation methods, prompts Snaith to declare that perovskite solar cells are poised “to break the prevailing paradigm” by combining low cost and excellent performance.
Snaith recently launched Oxford Photovoltaics, a start-up company, to commercialize this technology. The company is focusing on photovoltaic-based windows and architectural materials for buildings.
Similar to other relatively young photovoltaic technologies, perovskite solar cells can be fashioned using common wet chemistry techniques. The simplicity of making solar-cell components via liquid-phase chemical reactions and depositing the materials by methods such as spraying and spin coating may make it possible for solar-cell manufacturers to eventually replace clean rooms and sophisticated manufacturing equipment with simple benchtop processes, says Prashant V. Kamat, a professor in the University of Notre Dame’s chemistry department and radiation laboratory.

But even before manufacturers start building production facilities, the simplicity of perovskite solar-cell assembly is attracting many academic researchers. “Anyone can play,” Kamat says. The barrier to getting started in this field is low, he explains, because this type of research requires only standard lab equipment.
Another reason for the popularity is that perovskite photovoltaics hold much in common with—or can be viewed as an offshoot of—well-studied devices known as dye-sensitized solar cells (DSSCs). These devices are also called Grätzel cells after their inventor, Michael Grätzel of the Swiss Federal Institute of Technology, Lausanne. And DSSCs are closely related to yet another hot solar-cell technology—quantum dot solar cells (C&EN, Sept. 23, 2013, page 28). A key difference among these three cell types is the nature of the light absorber, which can be a trihalide perovskite, a dye, or inorganic quantum dots.
DSSCs feature a porous network of TiO2 particles that have been treated with a sunlight-absorbing dye, often a ruthenium compound, and typically infused with a liquid redox-active electrolyte material. As dye molecules absorb light and become energetically excited, they inject electrons into the TiO2 particles, which shuttle the electrons toward one of the cell’s electrodes. The molecules return to their original state via electron transfer from the redox material, which conducts positive charges, or “holes.”
In all of these types of solar cells, photoinduced excitation of electron-hole pairs and charge-transport processes work in concert to drive electrons toward one electrode and holes toward the other. These events generate a flow of electric current that can be used as a power source.
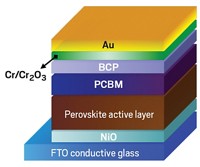
One of the shortcomings of DSSCs, especially early designs of these devices, is the thickness of the dye-coated TiO2 films. To absorb enough sunlight to do an adequate energy conversion job, the films tend to be several micrometers thick, which poses processing challenges, especially to efforts to make thin, flexible devices. That’s where trihalide perovskites come into play.
Northwestern University’s Robert P. H. Chang, a professor of materials science and engineering, explains that one of the main attractions of these materials is that they absorb sunlight more intensely and over a broader region of the solar spectrum than other light absorbers commonly used in solar cells. As a result, perovskite-based solar cells can be thinner than other types of cells, easier to process, and potentially less expensive to manufacture.
Some of those features, which now are widely known, were uncovered in a 2009 study by Tsutomu Miyasaka, an electrochemist at Toin University of Yokohama, in Japan, and coworkers. By treating a film of TiO2 with a solution containing CH3NH3I and PbI2, the team triggered a self-assembly process that coated the oxide with a layer of CH3NH3PbI3 nanocrystals, one of the perovskite materials at the center of current research efforts. They also prepared the tribromide from the corresponding brominated reagents.
The group fashioned solar cells by sandwiching the perovskite-coated oxide films together with an organic electrolyte solution between conducting glass electrodes. They found that the triiodide cell readily generated electric current with a conversion efficiency of 3.8%. The tribromide’s performance was a little weaker—3.1% (J. Am. Chem. Soc. 2009, DOI: 10.1021/ja809598r).
The performance was modest, but it attracted the attention of Nam-Gyu Park, a chemical engineering professor at Sungkyunkwan University, in South Korea. By optimizing various parameters, including the nature of the TiO2 film surface and the perovskite nanocrystal size, Park’s team bumped up the conversion efficiency of the triiodide cell to 6.5% (Nanoscale 2011, DOI: 10.1039/c1nr10867k).
But there was a hitch. These cells depended on an electrolyte solution that remained liquid in the finished device. Researchers had been trying for years to avoid using these liquids because they dramatically shorten cell lifetimes.
A common example of these materials, an organic solution of the iodide/triiodide (I–/I3–) redox couple, a hole conductor, is corrosive and volatile and quickly decomposes trihalide perovskites. As a result, these perovskite cells worked well, but only briefly. Scientists had shown previously that noncorrosive solid electrolytes could replace their troublesome liquid-phase counterparts. But the efficiencies of solid-state DSSCs tended to be low.
Help came in the form of a hole-conducting polyaromatic ring compound in the spirobifluorene family known as spiro-OMeTAD. With that compound on hand, things started to heat up quickly. Park teamed up with Grätzel and made solid-state spiro-OMeTAD/triiodide perovskite cells that exhibited much-improved stability and conversion efficiencies measuring 9.7% (Sci. Rep. 2012, DOI: 10.1038/srep00591).
And Snaith teamed up with Miyasaka, used spiro-OMeTAD as the hole-conducting layer, and deposited it on a mixed halide perovskite, CH3NH3PbI2Cl. When the team made solar cells with those compounds and TiO2, they observed conversion efficiencies near 8%, a value that just a year earlier would have turned heads. But when they replaced TiO2 with alumina (Al2O3), an insulator that cannot conduct electrons to the electrode—that is, when they made a solar cell that was sure to fail—it surprisingly delivered a whopping 10.9% conversion efficiency (Science 2012, DOI: 10.1126/science.1228604).
The team proposed that alumina serves only as a high-surface-area scaffold but that it mediates formation of a layer of high-quality perovskite crystals. The group suggested that the quality of the film is likely the reason the crystals can collect and transport electrons so efficiently.
Around the same time, Chang and Northwestern colleague Mercouri G. Kanatzidis, a chemistry professor, also reported success with a solid-state solar cell featuring a perovskite material. The cell was made from dye-coated nanoporous TiO2 and a novel light-absorbing, hole-conducting material with the perovskite structure—fluorine-doped CsSnI3 (also an ABX3 material). In tests of that cell, the group measured conversion efficiencies of 10.2% (C&EN, June 11, 2012, page 40).
In quick succession throughout 2013, one research paper after another popped up on journal websites, each describing a perovskite solar-cell design with a twist and each reporting efficiency improvements. For example, Sang Il Seok of South Korea’s Korea Research Institute of Chemical Technology (KRICT) found that by using polytriarylamine as a hole conductor, he and his coworkers made cells that achieved 12% conversion efficiencies (Nat. Photon. 2013, DOI: 10.1038/nphoton.2013.80).
And University of Saskatchewan chemists Dianyi Liu and Timothy L. Kelly did away with the high-temperature processing step necessary for conditioning TiO2 by replacing that oxide with a substantially thinner layer of ZnO. The team took advantage of those improvements to make flexible trihalide perovskite solar cells with conversion efficiencies in excess of 10%. They also made rigid cells that achieve efficiencies as high as 15.7% (Nat. Photon. 2013 , DOI: 10.1038/nphoton.2013.342). The highest efficiency perovskite solar cell verified to date by the National Renewable Energy Laboratory, which is regarded internationally as the official verifier of solar-cell performance, comes from KRICT and clocks in at 16.2%.
As researchers strive to increase conversion efficiencies, they also keep busy measuring, studying, and improving other cell parameters. “The materials in these cells are complicated, and we don’t really understand how they work,” Kanatzidis says. So his group has undertaken a detailed survey of phase transitions, charge transport, and other basic properties of lead and tin perovskites (Inorg. Chem. 2013, DOI: 10.1021/ic401215x). The group is currently working on ways to improve the chemical stability of tin compounds so they can serve as durable alternatives to lead-based perovskites.
Advertisement
Meanwhile, longtime photovoltaics researchers David Cahen and Gary Hodes, both professors at Weizmann Institute of Science, in Israel, are exploring ways to boost a key solar-cell parameter known as open-circuit voltage, VOC. Devices with high VOC values can capture high-energy photons and thereby exploit a broader range of the solar spectrum than can traditional cells. They also can be used to tap sunlight to directly drive electrochemical reactions.
By carefully matching the methyl ammonium lead tribromide perovskite with a hole conductor featuring handpicked electronic and optical properties, Cahen, Hodes, Eran Edri, and Saar Kirmayer made a solar cell with an open-circuit voltage measuring 1.3 V. That value is substantially higher—by several hundred millivolts—than unoptimized perovskite cells (J. Phys. Chem. Lett. 2013, DOI: 10.1021/jz400348q). Just recently, the team bumped up the VOC value further, to more than 1.5 V, by using a custom-made mixed bromide-chloride lead perovskite.
Unlike many other types of photovoltaics, in perovskites, it’s all about the chemistry, Hodes stresses. Precursor synthesis, cell assembly, and device customization are all mediated by ordinary chemistry methods, he adds, underscoring the prominent role chemists can play in this field.
Almost overnight, researchers catapulted the conversion efficiency of perovskite solar cells from a few percent to more than 16%. “It’s tough to predict where this technology will end up,” Snaith says, but it certainly has “the right ingredients” to deliver exceptional efficiencies at the lowest possible cost.
He adds, “So long as we can improve the stability of this technology, I would say we are witnessing the emergence of a contender for ultimately low-cost solar power.”
For a close-up look at the way perovskite solar cells are made, check out http://cenm.ag/perov.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter