Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Shape-Shifting Scaffold Supports Growing Bone Cells
Materials Science: A shape memory polymer could one day be used to help broken bones heal
by Melissae Fellet
February 13, 2014

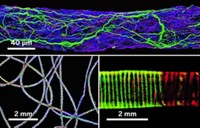
Researchers have used thin fibers of a shape-changing polymer to create a scaffold for growing bone tissue in the laboratory (ACS Appl. Mater. Interfaces 2014, DOI: 10.1021/am405101k). Such a material could help plug the holes left behind after the screws and plates used to repair broken bones are removed.

Broken bones held together by metal plates and screws are vulnerable to further damage because the difference in stiffness between the metal screw and the bone tissue weakens the bone near the screw. Yanzhong Zhang of Donghua University, in China, and his colleagues imagined reinforcing these surgically altered bones by removing the screws and filling the holes with a material that supports bone cell growth. The researchers also wanted to improve tissue regeneration by using a material that could generate mechanical forces to stimulate that growth.
As a first step toward that goal, the researchers made a biodegradable polymer scaffold and demonstrated that bone cells could grow and function on it. They chose a polymer made of a mixture of
The researchers used electrospinning to create thin fibers of a polymer containing an 8:2 ratio of
To test the polymer’s shape memory properties, the researchers spun the fibers into mats that they rolled into a cylindrical bar. The researchers warmed the plug to 39 °C, slightly above the temperature at which the polymer transitions from a rigid glass to a rubberlike material. They bent the warmed plug into the shape of an S and fixed the shape by cooling the bar to room temperature. The bar recovered its initial straight shape within 12 seconds of being rewarmed to 39 °C.
To test if the polymer scaffolds supported growing bone cells, the researchers seeded mats of the polymer with osteoblasts from newborn mice. The cells migrated, spread, and adhered to the mats faster than cells growing on smooth pieces of glass. The cells on the polymer scaffold also secreted minerals and produced an enzyme enhanced during bone formation.
Aaron S. Goldstein of Virginia Tech says bone engineering is a fairly new application for electrospun shape memory polymers. “Ultimately, it might be interesting to see how the rate and extent of bone healing compares between a deployed electrospun fiber material and an injectable gel or foam,” he says.
Patrick T. Mather, a biomedical engineer at Syracuse University, says that while bone cells appear to function on this scaffold, it is not clear if triggering a deformed scaffold to return to its original shape would affect the rate of mineral formation.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter