Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Chemical Sensing
Low-cost, Reusable Glucose Sensor
Biosensors: Nanoparticle-studded hydrogel detects glucose levels in urine with a simple color change
by Prachi Patel
June 2, 2014
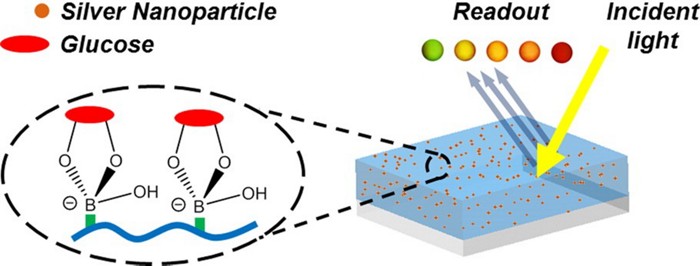
A simple, reusable glucose sensor could lead to a low-cost test for diagnosing and monitoring diabetes in clinics (Nano Lett. 2014, DOI: 10.1021/nl5012504). The device, which consists of silver nanoparticles embedded in a hydrogel, can detect glucose in urine samples just as accurately as commercial tests. But unlike those tests, it can be used hundreds of times, and its color-change readout is easy to interpret.
Currently, people with diabetes have two ways to measure glucose concentrations. One is the blood-glucose meter that requires expensive disposable test strips. The other, a urine dipstick test, requires spotting subtle color differences on the stick.
Ali K. Yetisen and his colleagues at the University of Cambridge wanted to develop an alternative sensor that avoided these limitations. Their solution was a reusable hydrogel that changes color from green to red depending on glucose levels in a patient’s urine sample.
The researchers make the gel by first polymerizing acrylamide monomers functionalized with phenylboronic acid groups. Then they form 30- to 50-nm-wide silver nanoparticles in the gel and blast it with a laser beam. A mirror underneath the gel, which is slightly tilted, reflects the light, creating an interference pattern in the gel containing periodic high-energy and low-energy spots. At the high-energy spots, the light breaks the silver nanoparticles into 15-nm-wide particles. The result is a hydrogel containing uniform arrangements of silver particles that can diffract incoming light.
To measure glucose levels, the researcher places drops of a urine sample on the gel and shines white light on it. The glucose in the urine binds with the phenylboronic acid groups, causing the gel to swell. This increases the distance between the silver nanoparticles, changing how the gel diffracts the incoming light. As the glucose concentration increases, the color of the diffracted light changes, and the gel goes from green to yellow to orange to red.
Rinsing the gel for 10 seconds with dilute acetic acid removes the glucose from the phenylboronic acid groups, making the sensor ready for reuse. The team could reset a single gel more than 400 times.
After calibrating the gel with urine samples containing known glucose levels, the scientists tested samples from 33 diabetic patients using the hydrogel method and a commercial urine dipstick test. The glucose measurements made by the hydrogel sensor were more accurate than those from the commercial test, as they more closely matched a standard electrochemical glucose measurement.
The sensor’s limitation so far is that scientists must slightly increase the urine’s pH to ensure glucose binds to the phenylboronic acid groups, Yetisen says. But he thinks they could avoid that step by tweaking the gel’s chemistry.
One of the technology’s advantages, Yetisen says, is that it should be inexpensive because the gels could be made on a large scale using the laser-based fabrication method. And clinics with limited resources could read out the measurements using smartphone cameras. Yetisen hopes to broaden the technology to detect electrolytes and enzymes in blood that are linked to kidney and liver disorders.
Advancing this sensor to work with blood rather than urine also would make it more useful for diabetic patients, says Sanford A. Asher, a chemist at the University of Pittsburgh. “You have to be very hyperglycemic to have glucose in your urine, so testing for glucose in urine has a limited ability,” he says. Nevertheless, he adds, “this is good work, and the science and engineering are strong.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter