Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Single-Enantiomer Dearomatization Tool Set For Chiral Action
by Stephen K. Ritter
March 9, 2015
| A version of this story appeared in
Volume 93, Issue 10
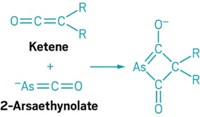
If Spanish architect Antoni Gaudí had designed a transition-metal complex, it would have looked like [tris(pyrazolyl)borate]W(NO)[P(CH3)3](η2-benzene). As gaudy as this molecule appears, chemists have found it to be a versatile synthetic tool for binding aromatic molecules such as phenols, pyridines, pyrroles, and naphthalenes and dearomatizing them, a key step in building the core structures of natural products and other bioactive compounds. One drawback to the tungsten reagent is that it’s prepared as a racemic mixture, so the products obtained using it are racemic mixtures that must be separated before continuing. A research team led by W. Dean Harman of the University of Virginia has solved that problem by devising a method to isolate the (R) and (S) versions of the tungsten complex so they can be used in enantioselective dearomatizations (J. Am. Chem. Soc. 2015, DOI: 10.1021/jacs.5b00490). The researchers first protonated the dimethoxybenzene analog of the tungsten complex with L- or D-dibenzoyltartaric acid, which enabled them to selectively precipitate a single diastereomeric salt of the complex. They then redissolved and deprotonated the complex and exchanged the dimethoxybenzene ligand for benzene to obtain the tungsten complex in its pure (R) or (S) form.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter