Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Sleep Aid Has Antituberculosis Activity
Ambien’s imidazo scaffold makes good starting point for finding novel antituberculosis agents
by Celia Henry Arnaud
January 12, 2015
| A version of this story appeared in
Volume 93, Issue 2
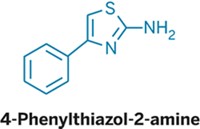
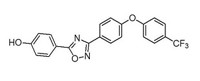
Zolpidem, better known as the sleep aid Ambien, is structurally similar to imidazo compounds that are promising antituberculosis agents. Marvin J. Miller of the University of Notre Dame and coworkers decided to see whether zolpidem also has anti-TB properties (ACS Infect. Dis. 2014, DOI: 10.1021/id500008t). Zolpidem itself is a mediocre inhibitor with, at best, a minimum inhibitory concentration (MIC) of 10 μM. But when the chemists rearranged the pieces of zolpidem into structural isomers, the MICs improved greatly, with one compound having an MIC of 0.004 μM. The researchers further elaborated the isomers into a series of analogs. In all, the team tested a total of 15 compounds against clinically relevant strains of drug-sensitive, multi-drug-resistant, and extensively drug-resistant Mycobacterium tuberculosis. Assays with a variety of microbes reveal that the compounds are selective for M. tuberculosis. So far, the compounds have been tested in cell lines but not in animal models. Studies to select optimal compounds are ongoing with scientists at Notre Dame, the pharmaceutical company Eli Lilly & Co., and the Infectious Disease Research Institute in Seattle, Miller says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter