Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Making Glucosepane In Eight Steps
Synthesis: Enantioselective route to protein adduct promises to help scientists better understand this moiety’s role in diabetes and aging
by Bethany Halford
October 19, 2015
| A version of this story appeared in
Volume 93, Issue 41
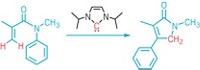
Some proteins are known to react with the open-chain forms of carbohydrates, forming adducts that have been linked to diabetes, inflammation, and aging. When this reaction happens between proteins and glucose, the moiety formed is called glucosepane. Scientists think there’s a direct relationship between glucosepane and symptoms of diabetes. But studying glucosepane has proven problematic because it’s difficult to isolate the adduct from biological samples and the glucosepane exists naturally as a mix of eight diastereomers. Thanks to a concise enantioselective synthesis devised by Yale University chemists David A. Spiegel, Cristian Draghici, and Tina Wang, scientists can now make and study all of these glucosepane isomers (Science 2015, DOI: 10.1126/science.aac9655). The key step in the eight-step synthesis is a one-pot procedure for making the nonaromatic 4H-imidazole tautomer. Because the synthesis is short and modular, the Yale chemists believe it will find many applications. For example, the synthesis could be used to incorporate glucosepane into synthetic oligopeptides, or it could help identify novel therapies for breaking glucosepane cross-links.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter