Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Catalysts Need Proper Support To Clean Up Engine Emissions
Pacifichem News: Phosphates help rhodium catalysts strip out NOx from exhaust
by Mitch Jacoby
December 16, 2015
| A version of this story appeared in
Volume 93, Issue 49

If automobile manufacturers had magic wands, they probably would wave them to simultaneously increase a car’s fuel efficiency and reduce its engine emissions. That would be a welcome trick because improving one often comes at the expense of the other.

No one was handing out magic wands at the 7th International Chemical Congress of Pacific Basin Societies, or Pacifichem, in Honolulu. But on Tuesday at a symposium focusing on automobile emissions cleanup, Haris Buwono, a graduate student working with Masato Machida of Kumamoto University, described a catalytic “magic trick” that could help carmakers sidestep the trade-off between fuel efficiency and emissions.
Burning gasoline in excess oxygen can boost fuel efficiency compared with burning stoichiometric mixtures of oxygen and fuel. But in the excess-oxygen condition, which is known as lean-burn because the air-to-fuel ratio is lean in fuel, today’s catalytic converters struggle to scrub nitrogen oxides (NOx) from the exhaust. So automakers design gasoline engines to operate near stoichiometry to reduce emissions of NOx, which are involved in reactions that produce ozone and smog in the atmosphere.
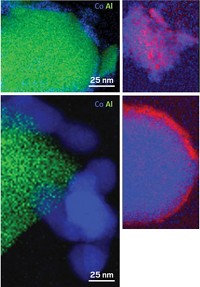
Machida reported that rhodium nanoparticles, the main catalytically active material in modern catalytic converters, can do a better job tackling NOx under lean conditions if the catalyst support is tailored for the job.
Working with Yuki Nagao, a catalyst specialist with Mitsui Mining & Smelting, and others, Machida prepared rhodium catalysts supported on a series of phosphates including ZrP2O7, LaPO4, AlPO4, and YPO4. The team tested these supported particles on various mixtures of NO, CO, propene, oxygen, and other gases, which they tailored to simulate exhaust gas mixtures corresponding to a range of air-to-fuel ratios.
Zirconium phosphate showed other promising features as a catalyst support. For example, Machida and coworkers found that excess oxygen readily oxidizes rhodium supported on ZrO2 to Rh2O3, a material with relatively low catalytic activity. When supported on ZrP2O7, however, rhodium resists that type of deactivation.
The researchers found that as the air-to-fuel ratio increased above stoichiometry, all of the phosphate-supported rhodium catalysts did a better job of scrubbing NOx than Rh/ZrO2, the conventional catalyst. Rh/ZrP2O7 worked best, removing about 35% more NOx than Rh/ZrO2 at fuel-to-air ratios slightly higher than stoichiometric (ACS Catal. 2015, DOI: 10.1021/cs5020157).
In addition, propene, an exhaust component resulting from incomplete fuel combustion, forms a reactive aldehyde on Rh/ZrP2O7. On ZrO2 it forms a less active carboxylate compound. The aldehyde helps clean up exhaust because it strips oxygen from NOx. That process reduces NOx to N2 and oxidizes the aldehyde to CO2 and water.
The results are clear, says Seoul National University’s Do Heui Kim, an emissions catalysis specialist: ZrP2O7-supported rhodium enhances removal of NOx from lean-burn exhaust. Kim adds that in addition to studying catalysts in powdered form, the researchers also anchored them on the same type of porous ceramic honeycomb-like brick found in automobiles everywhere. Testing the catalysts under these real-world conditions makes it much easier to translate the results to practical applications, he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter