Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
ACS Meeting News: Chemists Grind Up New Crystalline Metal-Organic Framework From Amorphous Material
by Jyllian Kemsley
August 18, 2015
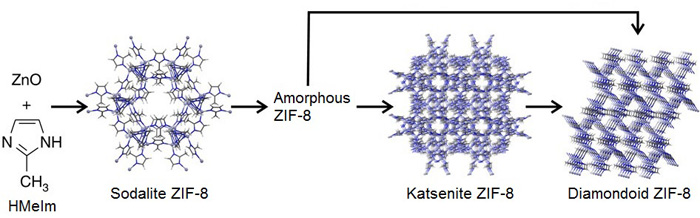
Grinding a crystalline metal-organic framework (MOF) material in a ball mill converts it into an amorphous structure and then into other crystal morphologies, according to research presented Tuesday at the American Chemical Society national meeting in Boston. The observation suggests researchers could use mechanochemical synthesis as a route to new MOFs.
Tomislav Friščić, a chemistry professor at Canada’s McGill University, reported the work in a symposium sponsored by the Division of Inorganic Chemistry. “We have almost direct proof of nucleation and crystal growth taking place during milling, which is very counterintuitive,” Friščić said.
MOFs are porous materials investigated for use in gas storage, catalysis, separation, and sensing. They are typically prepared from a metal salt and an organic ligand, using organic solvents in a process that often involves high temperature and bases. In contrast, mechanochemical synthesis replaces thermal with mechanical energy and can start from an easily obtainable metal oxide, eliminate the need for bases, and sharply reduce or eliminate solvents.
Friščić and colleagues, including Ivan Halasz of Croatia’s Ruđer Bošković Institute and Trong-On Do of Canada’s University of Laval, explored mechanochemical synthesis of a commercially relevant zeolitic MOF called ZIF-8 by grinding ZnO and 2-methylimidazole (HMeIm) using steel balls in a poly(methyl methacrylate) milling jar (Nat. Commun. 2015, DOI:10.1038/ncomms7662). The clear milling jar allowed them to monitor the material using an X-ray beam at the European Synchrotron Radiation Facility, in Grenoble, France. They added small amounts of acetic acid or water to the milling jar to catalyze the reaction.
While milling, they initially observed a diffraction pattern that they had expected: ZIF-8, Zn(MeIm)2, in the “sodalite” topology. The diffraction pattern, however, disappeared with further milling, indicating conversion to amorphous material.
Then another diffraction pattern appeared. “That was fully unexpected,” Friščić said. They eventually determined that the new diffraction pattern reflected a new ZIF-8 polymorph not previously observed. The team named it katsenite, after McGill postdoctoral fellow Athanassios D. Katsenis. Ground further, katsenite turns into a third, previously known diamondoid polymorph.
Mechanically synthesizing MOFs in a safer, cleaner way is a great advance, comments Omar Farha of Northwestern University. But seeing new topologies arise from amorphous material after grinding is “spectacular.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter