Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Engineering A New Chemical Communication System Into Bacteria
Synthetic Biology: Putting a synthetic quorum-sensing system into gram-positive bacteria could help improve industrial enzyme production
by Katharine Gammon
August 10, 2015

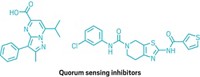
Many species of bacteria use quorum sensing, a type of chemical communications system, to direct behaviors—such as virulence, biofilm formation, and motility—on the basis of population density. Now, for the first time, researchers have engineered a synthetic quorum-sensing system in gram-positive bacteria, which could reduce the cost of producing proteins in these organisms (ACS Synth. Biol. 2015, DOI: 10.1021/acssynbio.5b00099).
Bacteria naturally use quorum sensing to coordinate gene expression according to the density of their local population. Engineering synthetic quorum-sensing systems into bacteria could allow scientists to turn on protein production at a certain time point during cell culture growth, enabling them to regulate gene expression without needing to add chemical inducers. In industrial production of proteins or biochemicals, this has the potential to improve processes and save cost.
Previously, synthetic biologists had only engineered synthetic quorum-sensing systems in gram-negative bacteria, such as Escherichia coli. But gram-positive bacteria are heavily used in the biotech industry to synthesize enzymes. So Cynthia H. Collins of Rensselaer Polytechnic Institute and colleagues wanted to build systems that would function within these commercially important bacteria.
It has been difficult to engineer cell-to-cell communication in gram-positive bacteria because many of the receptors for quorum-sensing signals used by synthetic biologists were engineered and isolated from gram-negative bacteria. “We needed new chemical wires to build new types of communication between a broader range of organisms,” Collins says.
The team genetically engineered gram-positive Bacillus megaterium to produce and secrete small peptides native to Staphylococcus aureus—a gram-positive bacterium with a well-characterized quorum-sensing system. A peptide-based quorum-sensing system has not been identified in B. megaterium, so adding the system elements from S. aureus ensured there would be no interference with natural signaling in B. megaterium, Collins explains.
B. megaterium was able to sense the peptides by expressing the receptors native to S. aureus. The cells produce signaling peptides and release them into the extracellular space, where they can then bind to receptors on the cell surface. That binding turns on a target gene—in this case, green fluorescent protein—in B. megaterium when the bacteria reach high cell densities.
“It’s an excellent paper,” says Michelle A. O’Malley, a chemical engineer at the University of California, Santa Barbara. “These sensing systems have been known for a long time in nature and in E.coli, but those platforms aren’t as translatable to industry as gram-positive bacteria, which can secrete protein-based by-products robustly.”
The researchers also took the idea one step further. They wanted to show that they could eventually create quorum-sensing systems involving two different types of gram-positive bacteria, allowing synthetic biologists to divide tasks between cells and engineer increasingly complex systems. So they split the signal production and sensing components of the quorum-sensing system between two strains of B. megaterium to produce cells that were either senders or receivers.
Kristala L. Jones Prather, a chemical engineer at Massachusetts Institute of Technology, says that the cell-to-cell communication in gram-positive organisms was particularly interesting. Although people have been writing about the potential of manipulating quorum sensing for industrial applications, “no one is doing this at any commercial scale,” she says. The challenge remains to have mixed organisms in a coculture maintain stability instead of outcompeting one another, Prather says, and this work represents a step forward.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter