Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Climate Change
Better Carbon Capture Through Chemistry
Climate Change: Researchers design materials to capture carbon dioxide emissions before they hit the atmosphere.
by Janet Pelley
December 7, 2015

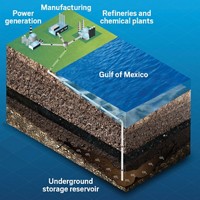
At Saskatchewan’s Boundary Dam power plant, not far from the U.S. border with North Dakota, one of its generating units burns some 800,000 tons of coal each year to provide about 139 MW of electricity to businesses and homes in the region. But since late 2014, the carbon dioxide produced by that burning has had a new fate: Instead of flitting up the smoke stack and out into the atmosphere, it gets trapped and compressed. The utility, SaskPower, then sends most of the CO2 along a pipeline to a nearby oil field where it is pumped underground to push oil out from below. About 2 km west of the plant, SaskPower injects the remaining portion 3.4 km deep into the Deadwood Formation, a brine-saturated sandstone formation, for permanent storage.
Janet Pelley is a freelance writer. A version of this story first appeared in ACS Central Science: http://cenm.ag/ccapture.
This snatching of CO2 before it escapes the plant and stuffing it away, known as carbon capture and storage (CCS), could decouple the burning of fossil fuels from its resulting climate-changing emissions. While some have criticized CCS as an environmentally and financially risky technology that props up the coal industry at the expense of research into renewables, most experts say it is a necessary tool for limiting climate change, and—like it or not—it will be widely deployed in the future. “The rate at which wind and solar power are growing is being outpaced by population growth and the rate at which energy demand is increasing,” says Christopher W. Jones, a chemical engineer at Georgia Tech. For the next 50 years, he says, fossil energy will supply the majority of society’s power needs. “If we really care about climate change, there is no choice but to implement CCS.”
The problem is that CCS is currently too expensive to be practical, so researchers are seeking new technologies to make it cheaper. Scientists are also working on ways to ensure the CO2 stays put after it is buried. Combined with the political will to reduce carbon emissions, these advancements could see much more carbon captured and stored in coming years.
Paris Climate Talks
A pivotal moment for climate change is here as nations are meeting in Paris for climate talks this week. In September this year, the world’s two largest greenhouse gas emitters, the U.S. and China, strengthened an earlier pledge to curb their carbon emissions, joining more than 150 other countries that have committed to do the same. This has raised hopes that the Paris meetings will produce a binding pact to cut greenhouse gas emissions, something previous climate talks have failed to achieve.

To cut future CO2 emissions enough to prevent catastrophic climate change, leading bodies such as the UN’s Intergovernmental Panel on Climate Change (IPCC) say CCS will be necessary; we cannot move away from coal fast enough to avoid emissions any other way. In its latest assessment report in 2014, the IPCC said that, without CCS, nations will have less than a 50% chance of staying below a global average temperature increase of 2 °C, a limit established by previous climate talks. To achieve this at the lowest cost, modeling by the International Energy Agency calls for 13% of needed emissions cuts to come from CCS.
Boundary Dam’s 139-MW unit now captures 1 million metric tons of CO2 per year, roughly 90% of its emissions, but it cost $1.3 billion. Despite the cost, the project went ahead because Canadian regulations require old burners to either convert to CCS or shut down. In addition, the company obtained a $240 million subsidy from the Canadian government and found a buyer for most of the CO2, notes Howard J. Herzog, a chemical engineer at Massachusetts Institute of Technology. “To justify the cost of CCS, you either need policy to force CO2 emissions reduction or government support or both,” he adds.
A fundamental barrier is the thermodynamics of the CO2 capture process, Herzog says. Today’s benchmark CCS technology, amine scrubbing, used in the Boundary Dam plant, consumes roughly 20–30% of the energy produced by a coal plant to capture and sequester its CO2 emissions, dramatically reducing a plant’s overall efficiency. During the process, CO2 bubbles up through a column filled with an alkaline amine solution, pulling the acidic CO2 molecules out of the air and into the solution. The CO2-laden liquid then flows to a heater, where the CO2 molecules evaporate from the mix and are trapped and compressed. Most of the energy used goes to heat the aqueous amine solution to 100–150 °C after scrubbing to regenerate the solution and capture the CO2. Water’s high heat capacity means this step takes a lot of energy.
A Solid Approach

To cut capture costs, scientists are turning to solid adsorbents, which have a substantially lower heat capacity than water. One such adsorbent was developed by Jones at Georgia Tech. He and his team incorporated solid particles made of polyethylenimine-silicon dioxide (PEI) into hollow plastic fibers about 1 mm in diameter, which they bundled into a tube before passing flue gases through it. The PEI particles adsorb CO2 through the same chemistry as liquid amines. Then to recover it, the scientists heat the fibers by running hot water down the center, releasing a stream of concentrated CO2 on the outside of the fibers. The high surface area provided by the fibers allows a lot of contact between gas and sorbent, Jones says. Also, the capture and release steps take place in the same vessel, making the process simpler and potentially cheaper than amine scrubbing, which requires a separate unit for each.
Meanwhile, the CCS community is abuzz over the potential of metal–organic frameworks (MOFs), porous structures built of metal ions linked by organic molecules. They have an enormous internal surface area, and those made of the right material can adsorb a lot of CO2, says Jeffrey R. Long, a chemist at the University of California, Berkeley. He and his colleagues designed magnesium- or manganese-based MOFs with diamine molecules loaded into the pores. When CO2 enters the cylindrical pores of the MOF, it binds to a diamine in a way that makes it easier and faster for the next CO2 molecule to bind the neighboring diamine molecule, promoting a chain reaction that quickly saturates the MOF. When the scientists raise the temperature by only 50 °C, the CO2 comes flooding off the framework.
MOFs are expensive, however, says Jennifer Wilcox, a chemical engineer at Stanford University. She and her colleagues have engineered a cheaper, carbon-based sorbent, similar to activated carbon, with embedded nitrogen functional groups and controllable pore structure that can be optimized to select for CO2. The material can be quickly cooled and heated. Also, CO2 nestles into the pores without forming a chemical bond, so it takes little energy to desorb the CO2.
Even better than sorbents could be membranes that separate CO2 from the flue gas, Wilcox adds, because then there’s no sorbent to regenerate and no water to heat. Gas separation membranes typically rely on a concentration or pressure difference across the membrane to drive the desired molecules through, but coal plant flue gas is only 14% CO2 by volume, too low to provide a driving force. Instead Wilcox and her colleagues created a membrane that is selective for the most abundant gas in power plant exhaust: nitrogen. The vanadium-based membrane allows nitrogen to pass, leaving CO2, water, and other trace gases behind on the other side.
All of these approaches are still at the research stage, but if any of them became practical, they could dramatically reduce the energy and cost penalties of CCS.
Still, researchers agree that what’s really needed to make CCS viable, regardless of the approach, is a price on carbon. Otherwise, why should a plant incur any cost if emitting CO2 to the atmosphere is free?
A Storage Problem

Carbon dioxide does, in some cases, have a value, and a hot area of research includes looking for ways to turn captured CO2 into valuable products that would offset the cost of capturing it. These run the gamut from carbonating beverages, making it into materials like plastics or concrete, feeding it to plants in enclosed greenhouses, or converting it back into methane or liquid fuel. But so far these are all niche applications or ideas at the research phase. “If you used CO2 for every product you can imagine, it would only consume about 20% of all the CO2 we produce,” says Ryan P. Lively, a chemical engineer at Georgia Tech. If any substantial fraction of the carbon emitted from coal plants is captured, for now, most of it will have to be buried.
Critics have raised perhaps the greatest concern about this step: If storage facilities leaked CO2 back into the atmosphere, it would undo the climate benefit from capturing it and tucking it away. But decades of oil and gas industry experience in enhanced oil recovery demonstrate that the storage technology works, says Grant Bromhal, an engineer at the Department of Energy’s National Energy Technology Laboratory.
For massive deployment of CCS, deep saline aquifers would likely be the major storage site. These reservoirs typically lie some 2–4 km below the surface of the Earth, composed of 50-m-thick, porous sandstone filled with saline water. The U.S. has enough of these formations to store 2,600 billion metric tons of CO2, sufficient for centuries of emissions. The CO2 stays put in such formations because they lie beneath impermeable shale, and capillary pressure in the sandstone pores locks the CO2 in place. Over time, the brine reacts with the CO2 to form solid calcium carbonate.
A Statoil natural gas plant in Norway has been storing 1 million metric tons per year in saline rock under the North Sea for 20 years with no evidence of leakage, but no one has tested projects as large as what a coal plant would produce—perhaps three to four times as much as the Statoil project. Uncertainties remain about how CO2 injected underground will travel and behave over time. DOE’s National Risk Assessment Partnership aims to study such concerns and develop best practices for CO2 injection, Bromhal says. For example, while fresh CO2-saturated brine readily dissolves cement in the lab, wells in the field have not degraded over four decades of use. The reason appears to be that the cement does not dissolve when the flux of brine over cement is intermittent. In fact, under these conditions, the cement changes the chemistry of the brine, leading to mineral precipitation and self-sealing of cracks. “We are now looking at ways to enhance the precipitation reaction in cements,” he says, which could help make storage safer.
Transitional Technology
Even if CO2 can be efficiently captured and safely stored, that does not mean countries should continue to rely on coal, which has other health and environmental impacts beyond climate change. But CCS can help society transition to a low carbon future, says Steve Clemmer of the Union of Concerned Scientists. And CCS is not just for coal plants, says Georgia Tech’s Lively. Industries such as cement and steel manufacturing produce large amounts of CO2 as part of the process chemistry. For these industries, the only way to avoid CO2 emissions is CCS.
North America now has 13 CCS projects either planned or in operation, launched as profit-making enhanced oil-recovery operations or as demonstrations with government funding. China, the world’s largest coal producer, is second in the world for CCS, with plans for nine large-scale projects. China is interested in CCS because it has numerous coal gasifiers that produce a large volume of CO2 that could be used to push oil out of the ground or as an industrial feedstock, says John Thompson, director of the fossil transition program at the Clean Air Task Force, an advocacy group. In 2011, the Shidongkou coal plant in Shanghai began capturing 100,000 tons of CO2 per year, which it sells to the beverage industry.
Advertisement
With the right outcome in Paris, experts agree that CCS could take off very fast. Technological improvements combined with commitments to make big cuts to carbon emissions—or a price on carbon to make cuts profitable—could allow the number of facilities to grow quickly. “The future of CCS will depend on political will to ensure deep, more than 50%, cuts in CO2 emissions,” says Simon J. Bennett, an energy analyst with the IEA. The Paris meetings will not emphasize any particular technology or specific strategies, but an agreement that is ambitious and acknowledges the role of innovation will support countries and companies developing CCS, he concludes. Then CO2 molecules around the world may share the fate of those at the Boundary Dam plant.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter