Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Phosphine ligand provides a new low for palladium catalysis
Sterically encumbered oxophosphole enables cross-coupling of complex aryl partners in water with a minimal amount of catalyst
by Stephen K. Ritter
March 3, 2016
| A version of this story appeared in
Volume 94, Issue 10
Palladium is the undisputed champion when it comes to catalyst metals, enabling chemists to carry out a versatile array of cross-coupling reactions. Bruce H. Lipshutz and his group at the University of California, Santa Barbara, have a goal to take this versatility further by seeing how green and efficient they can make palladium catalysis.
In their latest endeavor, the Lipshutz group and its collaborators have devised a new structurally optimized phosphine ligand that helps facilitate palladium-catalyzed cross-couplings with only a parts-per-million level of palladium, which is one to two orders of magnitude lower than that typically required (Angew. Chem. Int. Ed. 2016, DOI: 10.1002/anie.201510570).
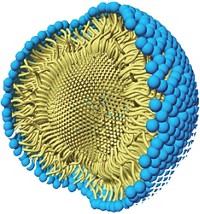
Chemists have concerns about the cost and availability of palladium and about the amount of residual palladium left after synthesis of a product requiring FDA approval. Lipshutz has been chipping away at these concerns in recent years by developing an aqueous nanomicelle reaction system that minimizes the amount of palladium needed. The lipophilic “nanoreactors” allow common organic reactions to be run in water at room temperature with repeated in-flask recycling of the nanomicelles and catalyst.
The new ligand, which is called HandaPhos after its creator, postdoc Sachin Handa, gives the researchers the ability to carry out Suzuki-Miyaura reactions in the nanomicelles using complex aryl coupling partners with less palladium than ever before, below 0.1 mol % compared with previous lows of about 1 mol %.
“Lipshutz is a magician who uses his micelles as a wand to make the impossible possible,” says Thomas J. Colacot, global R&D manager for homogeneous catalysis at Johnson Matthey Catalysis & Chiral Technologies. “The group’s work is having a great impact on our understanding of how Nature uses enzymes and trace amounts of metals to make biaryls, many of which are the essence of natural products.”




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter