Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Sustainability
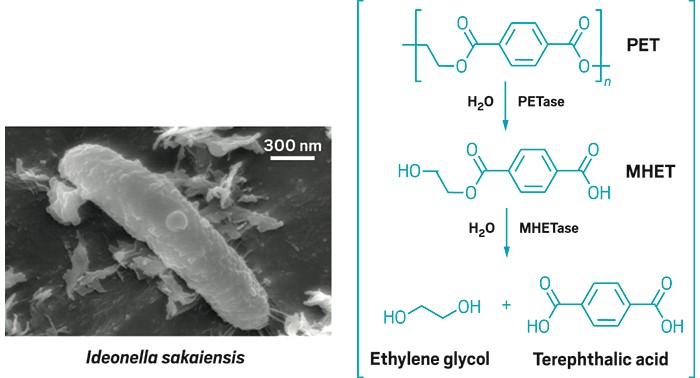
Bacteria feast on plastic
Microbes that munch on polyethylene terephthalate could be used to biodegrade this popular polymer
by Bethany Halford
March 10, 2016
| A version of this story appeared in
Volume 94, Issue 11

A tiny microbe one day could devour the millions of metric tons of polyethylene terephthalate, or PET, that pile up in landfills each year. Researchers in Japan have discovered the world’s first PET-eating bacterium, a critter that uses PET as its major carbon and energy source.
Each year, plastic manufacturers pump out more than 45 million metric tons of PET to make water bottles, salad domes, peanut butter jars, and other products—all of which sport a stamp with the number one inside a recycle symbol.
PET is the most recycled plastic in the U.S., according to PETRA, the PET Resin Association. But recycling rates still only reach about 31% nationwide. The European Union does better, recycling roughly half of its PET. Even so, tens of millions of metric tons of the plastic wind up in landfills each year, where the polymer’s strong ester bonds resist breakdown.
To find microbes that could pull PET apart, a team led by Kohei Oda of Kyoto Institute of Technology and Kenji Miyamoto of Keio University screened 250 sediment, soil, wastewater, and activated sludge samples from a PET bottle recycling facility in Sakai, Japan. After some careful microbial sleuthing, they found one bacterium that thrived on PET films and named it Ideonella sakaiensis after the city where it was found (Science 2016, DOI: 10.1126/science.aad6359).
PET can be hydrolyzed to its monomers chemically, but this process can be slow and usually requires high temperatures and pressures. Fungi that can break down PET have been identified previously, but the bacterium identified by Oda and Miyamoto’s group appears to be more efficient than these. In fact, I. sakaiensis dices up polymer at a surprisingly mild 30 °C.
The researchers further found that I. sakaiensis uses one enzyme, which they call a PETase, to break the plastic down into the intermediate mono(2-hydroxyethyl) terephthalic acid, or MHET. Another enzyme, dubbed MHETase, hydrolyzes the MHET into the monomers terephthalic acid and ethylene glycol. The scientists think this enzymatic machinery could one day remediate PET-contaminated environments or reclaim the plastic’s starting materials, which at present are derived from petroleum.
“This could provide huge savings in the production of new polymer without the need for petrol-based starting materials,” notes Uwe T. Bornscheuer, an enzyme catalysis expert at the University of Greifswald, in a commentary that accompanies the paper.
At the moment I. sakaiensis and its enzymes need some tweaking before they’re ready to chow down on the world’s PET waste. The bacterium prefers to dine on amorphous PET, rather than the crystalline PET used in products, and the enzymes work too slowly to be used industrially.
The study’s first author, Shosuke Yoshida of Keio University, says that a PET pretreatment that would enlarge the polymer’s amorphous areas would make waste more appetizing for the bacterium. Also, he notes, it might be possible to engineer the enzymes to make them faster and more practical.
This article has been translated into Spanish by Divulgame.org and can be found here.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter