Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Protein provides new route to carbon-silicon bonds
Bacterial cytochrome c demonstrates first example of biologically catalyzed organosilicon chemistry
by Jyllian Kemsley
November 24, 2016
| A version of this story appeared in
Volume 94, Issue 47

Silicon is the second most abundant element in Earth’s crust after oxygen, but carbon-silicon bonds are unheard of in nature: Neither biological organosilicon compounds nor biosynthetic pathways to create them have been identified. But when given the right starting materials, some heme proteins can stereospecifically form carbon-silicon bonds, report researchers from Caltech (Science 2016, DOI: 10.1126/science.aah6219).
“Nature’s iron heme chemistry just jumps on this opportunity because we provided it with the right precursors,” says Frances H. Arnold, who led the work with S. B. Jennifer Kan. “It’s a profound demonstration of how easily nature can innovate.”

“This closes a crucial gap between biological and chemical catalysis,” writes Martin Oestreich of the Technical University of Berlin in a commentary accompanying the paper. “The impact is unforeseeable, but it seems that we are a big step closer to potentially facilitating industrially relevant reactions such as alkene hydrosilylation with biomolecules.”
Chemists have experimented for decades with forming chiral organosilicon compounds by carbenoid insertion into Si–H bonds, but the reactions typically require halogenated solvents and catalysts made of precious metals coordinated with chiral ligands. The catalysts also have low turnover—each catalyst complex survives fewer than 100 reactions before it inactivates.
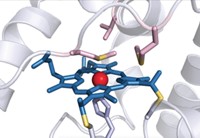
Prior work in Arnold’s lab and elsewhere had demonstrated that heme proteins can catalyze nonnatural carbene transfer reactions through insertion into N–H and S–H bonds. In the new experiments, the researchers screened a panel of heme proteins to find ones that could catalyze insertion of ethyl 2-diazopropanoate into the Si–H bond of dimethyl(phenyl)silane. Cytochrome c from the bacterium Rhodothermus marinus, which is found in submarine hot springs in Iceland, catalyzed the reaction with 97% enantiomeric excess, although the catalytic turnover was still low.
Cytochrome c proteins normally don’t catalyze chemical reactions; instead they transfer electrons between biomolecules in cells. But that did not stop Kan, Arnold, and colleagues from pushing the R. marinus cytochrome c to improve its newfound ability to perform organosilicon catalysis.
The researchers used directed evolution to come up with a set of three mutations that together increase the new enzyme’s enantioselectivity to greater than 99% and turnover to greater than 1,500. One of the mutations involved a methionine residue that provides an axial ligand to the protein’s iron center. A change from methionine to aspartic acid possibly provided more substrate access for the formation of an iron-carbenoid intermediate.
The “evolved” cytochrome c performs stereoselective carbene insertion into Si–H bonds using a variety of silicon and diazo reagents, without competing cyclopropanation. And when given 4-(dimethylsilyl)aniline, which can accommodate insertion at both N–H and Si–H bonds, the enzyme formed the organosilicon product with 97% chemoselectivity. Arnold thinks that the partially exposed active site promotes substrate promiscuity but that there’s also some characteristic of the heme pocket that prefers silicon chemistry.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter