Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
A New Life For Coal Ash
Sustainability: Electric utilities, environmentalists, researchers, and regulators converge on sustainable solutions for recycling waste from coal-fired power plants
by Stephen K. Ritter
February 15, 2016
| A version of this story appeared in
Volume 94, Issue 7

In December 2008, a dike collapsed at a waste storage pond at the Tennessee Valley Authority’s Kingston power plant in northeastern Tennessee. The incident released more than 3.8 billion L of water containing 4.1 million m3 of coal ash, which is the cremated remains of burning coal. The spill inundated several homes and contaminated the Emory River. The cleanup, which took until 2015 to complete, cost $1.1 billion.
The Kingston incident points to modern society’s biggest dilemma: In pursuit of our greatest need—generating electricity—we generate an unsustainable amount of pollution. In the case of burning coal, we regularly lament the amount of carbon dioxide being pumped into the atmosphere. But we tend to forget that other substances emitted by burning coal—including sulfur, mercury, and coal ash—are piling up on the ground.
Coal ash is the second-largest waste material in the U.S. behind household trash. Utility companies and the ash management firms working for them struggle to find economic ways to get rid of it. In the U.S., about half of the material is recycled in useful applications such as making concrete and gypsum wallboard. But the volume of coal ash produced and the economics of handling it is such that the other half must be disposed of as waste.
That means keeping it in large storage ponds like the one at Kingston or buried in landfills, which if not managed properly can leak out contaminants over time and cause an array of environmental problems. Power companies, environmental groups, scientists and engineers, and legislators and regulators have converged to come up with mutually acceptable solutions.
But finding such solutions has proved difficult. On one hand, anticoal environmental advocacy groups say coal ash poses unacceptable health and environmental risks and the best solution is to treat it as hazardous waste and lock it all away in lined ponds and capped landfills. The alkaline ash contains small amounts of toxic dioxins and polyaromatic hydrocarbons, along with traces of toxic metals—substances that could end up in drinking water.
On the other hand, utility companies say the best solution for the disposal problems, which will also help manage their operating costs, is to quit throwing coal ash away. Instead they want help finding affordable, value-added uses for the material.
The Environmental Protection Agency has been caught in the middle. EPA has spent decades evaluating coal ash safety. Sparked by the Kingston disaster, the agency developed a new set of rules that went into effect in October on coal ash management under the Resource Conservation & Recovery Act, which is the nation’s primary law on handling solid waste.
The rules continue to regulate coal ash as nonhazardous solid waste as before. In tests, contaminants in the ash rarely breach federal hazardous waste criteria, such as exceeding safe levels for drinking water. What’s new is that the rules stipulate more stringent standards for handling and disposing of coal ash and clarify how the rules will be enforced. The results, so far, seem to have left all parties involved in the issue unsatisfied.
Marc Yaggi, executive director of the Waterkeeper Alliance, was blunt in his criticism. “How could EPA conclude that coal ash, which is loaded with carcinogens, including arsenic, cadmium, and chromium, is not a hazardous waste?” he said in a statement in response to the EPA announcement. “The rules fall far short of what is needed.”
“The regulatory uncertainty over the years has caused more ash to be disposed of rather than recycled,” counters Thomas H. Adams, executive director of the American Coal Ash Association (ACAA), an industry group. “It’s been more about political science than real science.”
Zooming In On Coal Ash
Download a PDF of the graphic above here.
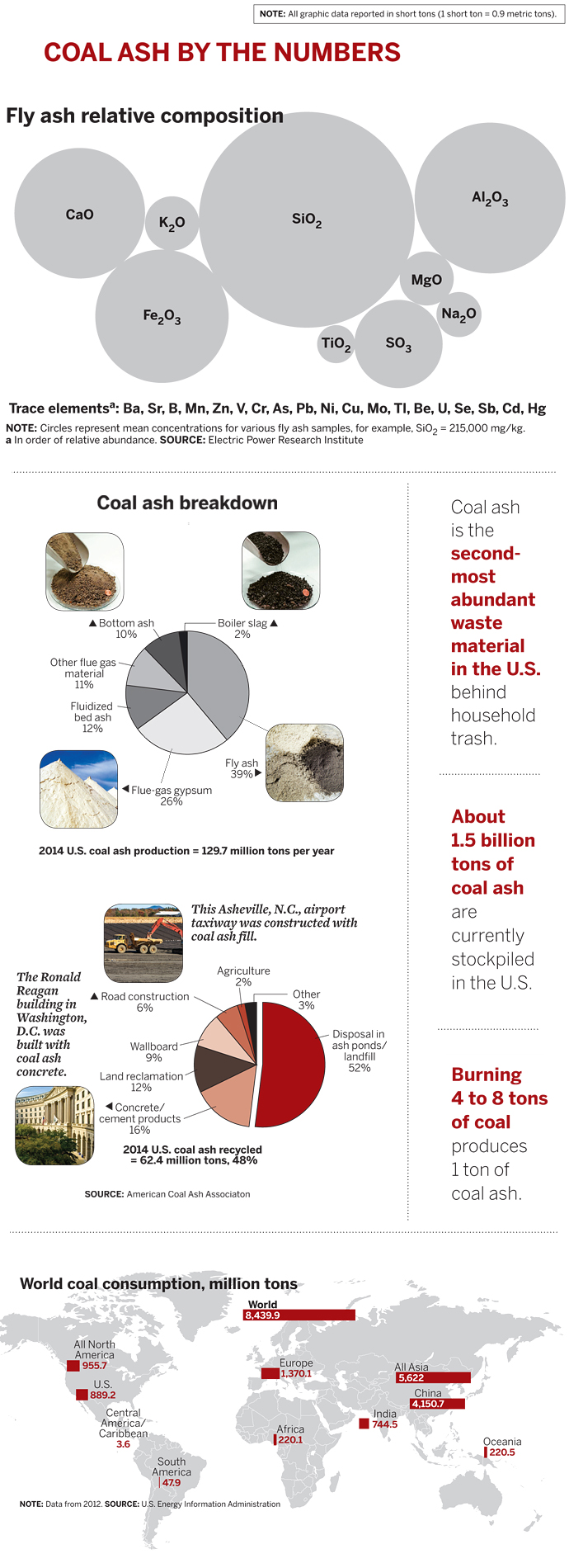
Coal ash is the mineralized residue left over from burning coal to generate electricity. It’s actually a collection of different types of materials, called coal combustion products or coal combustion residuals: fly ash, flue gas desulfurization products, bottom ash, and boiler slag. The type and amount produced depends on the kind of coal used and the type of furnace and combustion process.
According to ACAA, in 2014 U.S. coal power plants generated 129.7 million tons of coal ash. Of that amount, 62.4 million tons of the material, or 48%, was recycled. The remainder was disposed of in storage ponds or landfills.
Elsewhere in the world, coal ash management varies from country to country, ACAA’s Adams notes. In some European countries, such as Denmark, where there is no landfill space, all the ash is recycled. China provides subsidies to promote coal ash recycling, reaching recycling rates of 60%, according to the Asian Coal Ash Association. But the country produces about five times as much coal ash as the U.S., meaning a large volume of material is still treated as waste.
Scientists have poked and prodded coal ash for at least a century seeking remedies. Fly ash, the most abundant material, is a fine powder made up of particles that are trapped by electrostatic precipitators or fabric filters as flue gas from a coal furnace makes its way to a smokestack.
The ash is primarily made up of silicon, aluminum, calcium, and iron oxides, with lesser amounts of other metal oxides and sulfur. It also contains traces of mercury, cadmium, chromium, lead, arsenic, and other metals, as well as boron, nitrates, and fluoride. The composition is similar to common rocks and soils, as well as volcanic ash, which has been used as a construction material as far back as the ancient Romans.
Today, about half the concrete produced in the U.S. contains some fly ash—up to 40%—as a substitute for limestone-based portland cement. Among other applications, fly ash is used as material to make bricks, ceramic tiles, and plaster; as filler in metal and plastic composites and in paints and adhesives; and as structural fill for road construction.
Flue gas desulfurization products rank as the second most abundant type of coal ash. Utilities install equipment, commonly known as scrubbers, to trap sulfur oxides, nitrogen oxides, and other pollutants—emissions that contribute to acid rain and other environmental problems. In the case of sulfur, when the flue gas contacts a calcium-based sorbent such as limestone in a scrubber, the reaction forms hydrated calcium sulfate (CaSO4·2H2O), or gypsum. A host of other flue gas desulfurization materials are formed in various other scrubbers.
The synthetic gypsum is used like natural mined gypsum to produce wallboard, also known as drywall or Sheetrock. About half of wallboard manufactured in the U.S. is produced from power plant gypsum, according to ACAA. The recovered gypsum is also used in agriculture as a soil conditioner and to neutralize acidic soils.
Among other coal ash materials, bottom ash is a coarse material too large to float in the flue gas, so it falls through grates into a hopper in the bottom of the coal furnace. It’s typically used as filler in concrete and as fill material for road construction. Boiler slag is molten bottom ash that turns into pellets with a smooth glassy appearance after it is cooled with water. It’s useful as grit for sandblasting and polishing, roofing shingles, filler in asphalt, and a substitute for sand in snow and ice traction control.
Beyond these large-scale uses, there are few economic opportunities for recycling coal ash, Adams says. China extracts aluminum from coal ash. And the U.S. and China are also looking at whether it’s practical to extract other metals, such as rare-earth elements for electronics, uranium for nuclear power, and lithium for batteries—even gold. But these trace-metal extractions would require sifting through thousands of tons of ash to garner usable amounts of the metals, and the extracted ash would still remain.
Regulatory Dilemma
Since the 1980s, EPA has periodically issued reports concluding that coal ash is not hazardous and doesn’t need to be regulated as such. The agency has set up guidelines for handling coal ash as solid waste, similar to household trash, and has encouraged recycling. How the waste is actually handled is left up to individual states, subject to EPA approval.
The regulatory process has been fraught with contentious debate, as utility companies and environmental advocacy groups have sought the upper hand in their arguments for and against coal ash. The points of conflict include the hazardous/nonhazardous designation and how the regulations should be enforced—at the federal level or the state level. The current laws governing coal ash management rely on so-called citizen lawsuits, in which individuals or groups who believe violations are occurring must go to court to force EPA, the states, or utilities to abide by the rules.
Meanwhile, coal ash has been piling up. According to EPA, unused coal ash in the U.S. is currently disposed of in more than 735 ponds averaging more than 50 acres in size with an average depth of 6 meters, and in more than 310 landfill sites averaging 120 acres in size and an average depth of 12 meters. According to ACAA, roughly 1.5 billion tons of coal ash are already socked away. As environmental incidents involving coal ash storage began increasing in frequency starting in the 1990s, EPA was increasingly pressed to act further. The 2008 Kingston spill was the straw that broke the camel’s back.
So after further study, last year EPA updated its coal ash rules. Aside from reinforcing the agency’s nonhazardous waste determination, and leaving the door open for recycling, the rules call for remediating existing ash ponds and landfills that fail to meet the new disposal standards. For example, sites without impermeable liners to prevent material leaching into groundwater will need to be closed. That means the millions of liters of water in ponds will be removed and treated, then likely diverted into nearby streams. The ash that remains will be shunted off into recycling applications or moved to new lined landfills.
Electric utilities welcome the new rules as helpful, because the rules will open the damper on recycling efforts, ACAA’s Adams explains. If coal ash were labeled hazardous, he says, utilities likely would forgo recycling and dispose of the material as waste to avoid liability for its use in new products.
Even so, the ability to use coal ash still depends on the quality of the material and the results of a cost-benefit analysis, Adams notes. On the plus side, anytime coal ash is used in lieu of a virgin natural material such as portland cement, it reduces a portion of the fossil energy required, pollution generated, and ecological burden to mine, transport, and process those materials. For example, for every ton of fly ash used in place of portland cement, about 1 ton of CO2 emissions is eliminated, Adams says, citing EPA estimates. That’s equivalent to about two months’ emissions from an automobile. But there’s a short leash on those benefits, Adams adds. Companies must invest in infrastructure to use coal ash in concrete and wallboard, he explains, and if a company has to transport ash more than about 80 km for structural fill applications, it sometimes isn’t worth the effort and the company must dispose of the ash instead.
Beyond the economics, fly ash is beneficial in construction and as structural fill for its physical properties. For example, its composition reduces the amount of cement and water needed to make concrete and produces denser concrete with improved mechanical and chemical properties that make it stronger and more durable. For example, fly ash can nearly double the life of a highway, according to ACAA. And for synthetic gypsum, the material made at power plants is typically higher purity than mined gypsum, making it preferable for wallboard.
“We have by no means hit the ceiling in terms of market potential for coal ash,” Adams says. “Now that we have regulatory certainty, investment will start moving back into the infrastructure we need to grow coal ash recycling,” he notes. “That’s good news for the coal ash industry.”
New Opportunities
One potential use for coal ash, ironically, is to help clean up old coal mines. Civil engineers Tarunjit S. Butalia and Chin-Min Cheng of Ohio State University are studying whether flue gas desulfurization materials could be used to mitigate acidic drainage from hundreds of abandoned coal mining operations in their state.
After a mine is abandoned, groundwater continues to percolate through boundaries in coal seams, picking up minerals, most commonly pyrite (FeS2), Butalia and Cheng explain. Once exposed to air, either in a tunnel underground or dripping from a high exposed mine wall aboveground, pyrite converts into ferrous iron (Fe2+) and sulfuric acid (H+ and SO42-), creating an acidic brew. If not contained, the drainage can seep into groundwater or into streams, harming the environment.
Advertisement
As part of Ohio’s ash management plan, Butalia and Cheng are focusing on high-volume applications for the claylike desulfurization materials, such as using it as a treatment filter to remediate mine drainage. They found in lab tests that as acidic mine water passes through the alkaline material, the water is neutralized and many of the dissolved trace elements adsorb or precipitate and are trapped. The water that comes out the other side isn’t perfectly clean, Cheng says, but the amounts of heavy metals of concern that remain are similar to those in streams or groundwater and typically below limits set for drinking water.
Butalia and Cheng are directing a test project in which some 500,000 tons of sulfite flue gas material from American Electric Power’s Gavin Power Plant will be used to sop up acid mine drainage at an abandoned coal mine near the Ohio River. A layer of the material some 300 meters long and up to 60 meters wide will be placed against the mine wall to serve as a barrier through which the drainage must pass. The researchers estimate the barrier will provide more than 50 years of treatment capacity.
The circular nature of what they are doing hasn’t escaped the Ohio State engineers. “The problems that arose before coal mining was regulated are now being addressed by using residues that come from burning coal,” Butalia says. “These abandoned mine sites are an eyesore, a safety hazard, and an environmental liability for the state. The coal-combustion by-products are the same for utility companies. By diverting material that would otherwise go to a landfill and instead using it to improve the environment, we are addressing two problems with a synergistic solution.”
Despite coal ash’s promise, the environmental concerns of using the material are not going away. There’s a case to be made for recycling coal ash into useful products, the Waterkeeper Alliance’s Yaggi tells C&EN. “But it’s our strong preference that coal no longer be burned to create electricity in the first place. Renewable energy has proven itself to be a viable alternative to dirty fossil fuels. That said, there is an enormous amount of coal ash, and current disposal methods leave waterways and communities at risk.
“We believe that encapsulated recycling of fly ash into concrete, if done properly, reduces the leaching of toxic materials and is the least environmentally problematic option,” Yaggi continues. Unencapsulated recycling options for coal ash, including use in structural and mine fill, or as fertilizer or other agricultural materials, “pose unacceptable risks that we cannot endorse.”
The Challenges Ahead
On Feb. 2, 2014, an aging storm drain ruptured at a coal ash pond at Duke Energy’s retired Dan River Steam Station in Eden, N.C. The equipment failure sent some 132 million L of water laden with 70,000 tons of coal ash into the Dan River during the course of a week, fouling 100 km of the waterway. Duke Energy, following its 2012 merger with Progress Energy, is now the U.S.’s largest electric utility company.
Duke Energy and its predecessors have been criticized for years by environmental groups in the region for their handling of coal ash, including being unresponsive to leaky ash ponds at multiple sites. The environmental groups have also been up in arms over what they consider lax oversight by the North Carolina Department of Energy & Natural Resources. Duke Energy and the state are now being forced to act, and their case exemplifies the challenges that lie ahead for coal ash.
In February 2015, Duke Energy was charged by federal authorities for violating the Clean Water Act and other regulations stemming from improper disposal of coal ash at multiple sites in North Carolina. The company entered a plea agreement to settle the matter with a $102 million payment. The company has $121 billion in assets.
To address the charges and to meet updated federal and state coal ash rules, Duke Energy has created a plan to close and remediate all 32 of its coal ash ponds in North Carolina, says company spokeswoman Catherine H. Butler. Deciding what to do with all the material isn’t a snap. Each site is unique, requiring a customized solution for dewatering ponds and removing the ash, all while protecting groundwater and the surface environment, Butler says. The company anticipates spending as much as $10 billion over 30 years for the remediation.
“As we develop our ash basin closure plans, our goal is to find opportunities to reuse that ash, rather than rebury it,” Butler says. “And we are staying focused on our operating plants to increase the amount of ash recycled from them, so it doesn’t have to be stored away.”
For example, 3 million tons of Duke Energy ash currently stored in basins is being removed by Charah, a construction company that specializes in coal ash management. The ash is being used as structural material in lined basins to fill in two open-pit clay mines used by brick manufacturers in central North Carolina. The remediated land could be repurposed for future development, perhaps as an industrial site. In another project, Charah is placing more than 4 million tons of ash in a lined structural fill at the Asheville Regional Airport to build a new taxiway. “These three projects allow for a large amount of ash to be recycled in a short time frame,” Butler says.
Duke Energy currently recycles 47% of the ash it produces at working coal power plants company-wide, but only 30% in North Carolina, both figures that the company aims to increase, Butler adds. Much of the coal ash goes to make concrete, she says. The company also has gypsum recycling operations at most of its operating coal plants. One exception is the company’s plant in Edwardsport, Ind., where 99.9% pure sulfur is extracted from the flue gas. Duke Energy sells the sulfur into the chemical and fertilizer markets.
“That’s a large portion of our ash that is not going into our ash basins,” Butler says. “But we’re aggressively exploring ways to safely reuse even more.”
As part of its state regulatory requirements, Duke Energy has engaged the Electric Power Research Institute, a nonprofit R&D firm, to conduct a study, expected to be completed midyear, to understand the market demand for recycling coal ash with current uses and to identify emerging technology opportunities.
A Duke Energy team is already researching additional means to make coal ash more suitable for recycling, Butler notes. But the company has to balance cost and regulatory requirements, she explains. For example, to meet restrictions on nitrogen oxide and other emissions and still provide cheap power to benefit customers, power plants are sometimes limited in the type of coal they use and how they burn it. The combustion therefore might be incomplete, leaving unburned carbon in the ash. That can be a problem, because less than 2% carbon content is required to make concrete. “If we can’t burn all the carbon out of the coal, the ash may not be suitable for recycling,” Butler says.
The company is pursuing thermal beneficiation technology that would burn out the carbon to an acceptable level, allowing for both new and old ash to be treated and used in concrete products, Butler says. But the added cost could tip the scales against recycling. She concedes: “It’s part of the push and pull of coal ash.”





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter