Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Nickel catalyst "chain walks" to produce fatty acids
Catalytic reaction adds CO2 to selected C–H bonds in alkane and alkene derivatives
by Stu Borman
May 3, 2017
| A version of this story appeared in
Volume 95, Issue 19

Many of the C–H bonds in hydrocarbons look alike to chemical reagents. As a result, synthetic chemists often have to install activating or directing groups to get reactions to occur at C–H bonds of their choosing.

In a new reaction, a nickel catalyst walks along hydrocarbon chains to activate specific C–H bonds for reaction, without the need for preinstalled activating or directing groups (Nature 2017, DOI: 10.1038/nature22316).
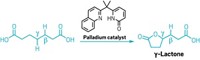
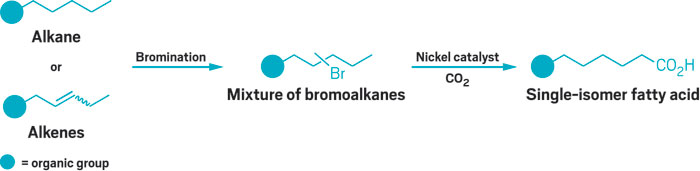
The method, developed by Rubén Martín of the Institute of Chemical Research of Catalonia and coworkers, provides a new route to fatty acids, which are used industrially to make soaps, detergents, rubber, plastics, and dyes. Each reaction produces a single-isomer fatty acid by adding CO2 to a specific C–H bond in a pure alkane or an alkene mixture derived from inexpensive petroleum feedstocks.
Industry currently makes fatty acids by hydrolyzing naturally derived lipids or by hydrocarbonylation of alkenes with carbon monoxide. Matthew Gaunt and Patrick Williamson of the University of Cambridge explain in a Nature commentary accompanying the new paper that those processes require separations and purifications to isolate specific fatty acids. The Martín reaction avoids those steps by creating specific regioisomers, “a remarkable feat of chemical selectivity,” Gaunt and Williamson write.
In the reaction, chemists first brominate a specific alkane or an alkene mixture. Either type of hydrocarbon can have an organic group at one end. Alkene molecules in each mixture must all have the same length chain, but the position of the double bonds can vary. The bromine also can add at any position in the alkane or alkene chains, as its location does not guide selectivity for CO2 addition. A nickel catalyst substitutes for bromine and “chain walks” its way to a selected C–H bond.
Chain walking exploits usually undesirable β-hydride eliminations that occur spontaneously in alkyl-halide substitution reactions. With each elimination, the catalyst moves one carbon from its current position and then takes steps down the chain until it gets to the target C–H site, where CO2 substitutes for it. Chain walking has been used before, but in less versatile ways and typically with expensive noble metal catalysts.
The chemists control site selectivity though the reaction temperature. At lower temperatures, kinetic control installs CO2 at the least hindered site, the alkyl terminus. At higher temperatures, thermodynamic control takes over, installing CO2 at selected interior sites. “There are a number of remarkable features in this work, but the most stunning aspect is the selectivity switch that’s controlled just by changing the temperature,” comments Olivier Baudoin of the University of Basel, who recently developed another chain-walking reaction.
“The goal of taking bulk commodity hydrocarbon feedstocks and converting them to chemicals of higher value is exciting, and rarely have I seen its full potential demonstrated as well as in this work,” says synthetic chemist M. Christina White of the University of Illinois, Urbana-Champaign. “This reaction goes beyond proof of concept and is immediately useful. The ability to exploit β-hydride elimination pathways that terminate in a favored site of functionalization will certainly inspire future reaction designs.”
CORRECTION: This story was updated on May 4, 2017, to correct a statement about how temperature controls which C–H site on a molecule gets targeted for CO2 installation. At low temperatures, the CO2 targets the alkyl terminus of a molecule rather than an interior C–H site. And at high temperatures, the opposite is true.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter