Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Analytical Chemistry
Enhancing carbon-13 NMR signals in liquids
Researchers improve effectiveness of dynamic nuclear polarization through choice of polarizer and other parameter changes
by Jyllian Kemsley
February 16, 2017
| A version of this story appeared in
Volume 95, Issue 8
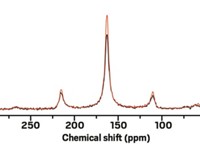
The power of nuclear magnetic resonance spectroscopy lies in its high resolution, while the technique’s weakness is its low sensitivity. One way to boost NMR signals is through dynamic nuclear polarization (DNP), in which microwave irradiation transfers spin polarization from electrons of a stable radical to the nuclear spins of interest. A research team has now demonstrated signal enhancement of two to three orders of magnitude for room-temperature carbon-13 NMR experiments (Nat. Chem. 2017, DOI: 10.1038/nchem.2723).
DNP has historically worked well in solid-state NMR experiments but struggled in liquids. Spectroscopists believed that molecular motions in liquids impeded the transfer of spin polarization at high magnetic fields. One work-around is to polarize the radical at low temperatures and then warm up the sample, but that approach limits the number of scans that can be taken for signal averaging.
The new work was conducted by a team at the Max Planck Institute for Biophysical Chemistry, led by Marina Bennati and Guoquan Liu, who is now at Peking University. The researchers found that one key to improving DNP-enhanced carbon-13 NMR of liquids at high magnetic fields was using a nitroxide radical as a polarizer.
After optimizing several parameters in their DNP-NMR protocol, including efficiently saturating the radical electron spin polarization, the team obtained significantly improved signals for molecules such as CCl4 and CHCl3, as well as for biologically relevant compounds such as pyruvate and ethyl acetoacetate. The experiments can be repeated within seconds for signal averaging.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter