Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Oocytes live longer than most cells in the body. How?
A recent study identifies a cleanup pathway that could help address this long-standing question but also answer some unknowns about fertility decline.
by Priyanka Runwal
March 4, 2024
| A version of this story appeared in
Volume 102, Issue 7
Oocytes are among the longest-lived cells. Scientists have been trying to understand what enables such longevity, because oocytes—like other nondividing, long-lasting cells—have a high risk of accumulating misfolded proteins. These protein aggregates, a common feature of aging, can be toxic to cells and impact egg quality.
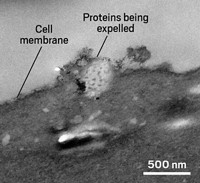
In a recent study, researchers in Europe described a cleanup pathway operating in young oocytes in mice (Cell 2024, DOI: 10.1016/j.cell.2024.01.031). The process involves collections of lysosomes and other protein-degrading cellular machinery that the research team describes as super-organelles. These assemblies temporarily store the aggregates until the oocytes start to mature. As the oocytes convert to ovums, these super-organelles begin to degrade the harmful proteins.
It’s the first time this major degradative route has been described in mammlian oocytes, says Gabriele Zaffagnini, a biochemist at Barcelona’s Center for Genomic Regulation and the study’s lead author. The findings could provide a new avenue of investigation for scientists studying fertility decline and infertility in women.
To understand how mice oocytes handle protein aggregates, Zaffagnini and his colleagues first used a fluorescent dye to stain clumps of misfolded proteins within oocytes. Next, they stained for a protein commonly used as a marker for lysosomes. The location of the misfolded protein clumps and the lysosomes perfectly overlapped in the oocytes, Zaffagnini says. But he noticed something unusual: each clump wasn’t contained in one large lysosome but within a tightly packed gathering of several lysosomes and other organelles involved in degradation. The team called this grouping endolysosomal vesicular assemblies (ELVAs), in which individual organelles worked together as one unit.
Zaffagnini and his colleagues noticed that these ELVAs were inactive in live oocytes—meaning that the assemblies were storing but not degrading the misfolded proteins. “We think this is an adaption to save energy,” he says. But when the oocytes start maturing, ELVAs get to work and start chopping up the aggregated proteins. The team found that this cleanup is essential for correct embryo development.
Coleen Murphy, a molecular biologist at Princeton University who was not involved in the research, says that the study is reminiscent of similar work involving the oocytes of the roundworm Caenorhabditis elegans. For her, the next step would be to repeat the Zaffagnini team’s experiments in aging oocytes from mice, particularly to understand mechanisms for fertility decline. That work has already begun, Zaffagnini says: early results suggest that in older oocytes the ELVAs may be dysregulated, throwing off the crucial cleanup timing. His team is also initiating the research in human oocytes, which live much longer than mice oocytes.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter