Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biochemistry
A surprising molecular mechanism for processing bitter tastes
Bitter taste receptor’s role goes beyond the tongue
by Sarah Braner
April 19, 2024
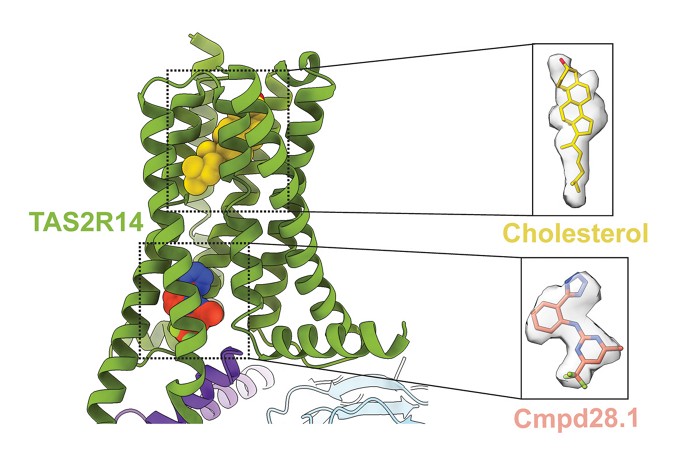
Researchers have uncovered the structure of a known, widespread human taste receptor, which may help scientists understand the role it plays outside the tongue.
“What we found was totally unexpected,” says Bryan Roth, one of the lead authors of the study published in Nature on April 10th (2024, DOI: 10.1038/s41586-024-07253-y). “We were actually kind of shocked when we got the structure.”
The orthosteric site on a receptor is typically the main site where a ligand would bind. This site is in contrast to the allosteric site, which is elsewhere on the receptor and plays more of a modulator role.
The Roth team found that the receptor, TAS2R14, does not bind tastants at the orthosteric site, which is where they would be expected to bind. They found that this site was highly hydrophobic, which would repel bitter tastants because they are positively charged.
Instead, they found that cholesterol would bind to that orthosteric site. Cholesterol itself does not seem to activate TAS2R14, but it seems to “prime” the receptor so that the receptor can respond when a bitter tastant binds to the allosteric site rather than the expected orthosteric site. Cholesterol also connects TAS2R14 to the G-protein it’s attached to, acting as somewhat of a “molecular glue,” Roth says.
The structure of TAS2R14, a G-protein-coupled receptor (GPCR) responsible for sensing bitter tastants, has been difficult to discover because this receptor does not look like other GPCRs, according to Roth.
“If you make a model based on homology with other GPCRs, then you’re going to be led astray,” he says.
TAS2R14 is also present outside the mouth and tongue; the team found that it was highly expressed in the cerebellum as well as organs involved in metabolism like the gut and liver. The fact that it is expressed outside the mouth isn’t new, but what is new is that cholesterol circulating in the body may play a role in the receptor’s functions outside the mouth—essentially, this orthosteric site could act as a sensor for intracellular metabolites.
Alex Woo, the CEO of the food innovation company W20 and an expert on the neuroscience of taste, says that the applications of these findings are still unclear, but he guesses that the findings could be used in drug development for heart disease down the line.
Cholesterol binding to TAS2R14 is just the beginning of a potential signal cascade, Woo says, “so the downstream signal of cholesterol binding has to be related to cholesterol metabolism in your body.” This new research shows the start of a signal pathway, but the end result is still unknown.
The Roth team is planning to follow up on their findings by looking for other taste receptors that may also function the way TAS2R14 does, along with finding metabolites that behave like cholesterol with these sites.
“It’s rare in this field that anything brand new appears. So this is completely mind blowing,” Roth says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter