Researchers have found a new molecular role for MeCP2, the protein that doesn’t work correctly in people with Rett syndrome. The findings could suggest new ways to treat the neurodevelopmental condition, the scientists say.
Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Epigenetics
Scientists identify a possible molecular mechanism of Rett syndrome
Epigeneticists stumble across link between methylated DNA repeats and protein involved in rare genetic disorder
by Laura Howes
June 24, 2021
| A version of this story appeared in
Volume 99, Issue 24
Rett syndrome is a genetic condition that mainly affects girls, and usually becomes apparent in children 6–18 months old. Those children with the syndrome regress developmentally, and lose motor control and the ability to speak. In 1999, Huda Zoghbi at the Baylor College of Medicine and colleagues determined that the disorder was caused by mutations in the gene coding for the protein MeCP2.
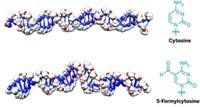
Ali Hamiche at the Institute of Genetics and Molecular and Cellular Biology in Strasbourg and his group found the new role for MeCP2 while investigating methylated and hydroxymethylated cytosine and adenosine repeats in DNA (CACACACACA, and so on). The group was looking for proteins that bind or interact with these genetic elements to understand how these sequences affect gene regulation. To the group’s surprise, Hamiche says, they found that MeCP2 binds these repeating sequences (Science 2021, DOI: 10.1126/science.abd5581).
Hamiche and his colleagues then teamed up with structural biologists and used X-ray crystallography to observe how mutated and nonmutated versions of the protein bind to DNA. The scientists found that when MeCP2 without Rett-related mutations binds to specific CA repeats, it changes the DNA structure. This change prevents DNA from being wrapped up into nucleosomes. In contrast, mutated MeCP2 proteins can’t bind the CA repeats and, as a result, these repeat regions have many more nucleosomes and end up much more tightly packed. This tight packing could potentially change the regulation of genes along that stretch of DNA.
In a commentary accompanying the new paper, Zoghbi explains that the findings back up previous evidence that MeCP2 is involved in the organization of chromatin, the cellular material consisting of DNA wrapped into nucleosomes. But Qiang Chang, who researches MeCP2 at the University of Wisconsin, Madison, says that the relevance of this newly discovered function for MeCP2 to Rett syndrome remains to be proven. MeCP2 has other functions in addition to DNA binding and more research is needed to show that this wrapping mechanism happens in the neurons of people with Rett syndrome and how it leads to the neurodevelopmental effects of the disease.
Hamiche hopes the team’s new findings can help in the search for a treatment. His team continues to work on how exactly MeCP2 affects chromatin reorganization and nucleosome binding.
Correction
This story was updated on June 24, 2021, to correct the description of the DNA repeats that MeCP2 binds. The protein binds repeats of cytosine and adenosine, not cytosine and arginine. The update also corrects the description of nucleosomes. These structures consist of DNA wrapped up onto histone proteins and are not proteins themselves.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter