Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy
Sweat could power wearables with nanogenerator
MXene-coated wool produces electricity from the flow and evaporation of sweat
by Prachi Patel
April 22, 2024
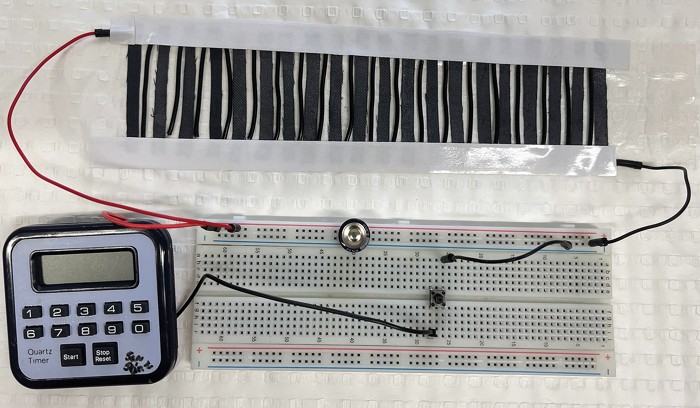
A new power generator could let people charge fitness trackers and smartwatches with their own perspiration (Device 2024, DOI: 10.1016/j.device.2024.100356). The thin, flexible generator, which could be easily embedded in a wristband or headband, harnesses energy from evaporating sweat and produces electricity.
The device is a type of hydroelectric nanogenerator (HENG). “Its working mechanism is pretty fascinating,” says Jingliang Li, a chemical engineer at Deakin University. HENGs have porous substrates loaded with functional materials that absorb water and split it into positive and negative ions, which line up along the substrate’s channels. As some water evaporates, capillary force drives more water past the charged channel walls, creating a movement of charges that generates an electric current.
Researchers have made similar generators before using carbon particles or nanotubes, but the output power has been low because the particles clump together. Li, Azadeh Nilghaz, and colleagues used MXenes, which are 2D layered transition-metal carbides or nitrides that are covered with functional groups.
The Deakin team made a HENG by dipping wool into a solution of single-layer titanium aluminum carbide MXene nanosheets. The sheets are hydrophilic, are conductive, have hydroxyl and carboxyl surface groups, and uniformly coat the wool fibers. All these factors help the HENG efficiently absorb water, split it, and move charges. With 0.6 mL of artificial sweat, a 40 cm2 HENG charged a commercial supercapacitor in 6 min, while a 400 cm2 carbon-based HENG took 90 min. Affixed to a volunteer’s T-shirt, the new MXene-based HENG charged a stopwatch after the wearer ran for 10 min.
The use of MXenes is key for high-efficiency HENGs, says Huhu Cheng, a chemistry professor at Tsinghua University. But the new device’s power is more intermittent than that of other HENGs. “That could be improved through further materials, device, and system design,” he says.
Nilghaz says the team is working on making the devices more efficient and practical for everyday use. The researchers are also combining the HENGs with biosensors and a Bluetooth communication device for a health-monitoring system.”





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter