Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pollution
One-two punch degrades antibiotics in wastewater
New material combines light-generated heat and catalysis to break down penicillin
by Prachi Patel
December 22, 2023

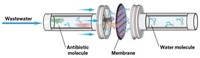
A new material rapidly converts sunlight to high temperatures and uses the heat to catalyze the breakdown of penicillin within 10 minutes (Proc. Natl. Acad. Sci. USA 2023, DOI: 10.1073/pnas.2302761120). Embedded in a membrane, the light-driven material should be easy to use at water treatment facilities and should also be effective on other similar antibiotics, says Wanhong Ma, a chemist at the Institute of Chemistry, Chinese Academy of Sciences who led the new work.
The rise of antibiotic-resistant bacteria is a pressing global health crisis. Wastewater, especially from drug companies and farms contains large volumes of antibiotics. Destroying these drugs could prevent pathogens from developing resistance, but it is difficult to do. Current methods usingadsorption and oxidation to break down antibiotics are “too expensive and difficult to operate, or ineffective,” says Ma. Some metal ions can trigger the breakdown of β-lactam antibiotics—the most common class of the drugs, which includes penicillin—via hydrolysis. But the process is too slow at room temperature to be practical. Heating the wastewater to speed up the process would consume a lot of energy.
So Ma, Dongge Ma, Hongwei Ji and colleagues loaded copper ions (Cu2+) ions into a covalent organic framework (COF). COFs are porous materials composed of a network of organic building blocks covalently linked together. Some COFs can convert light to heat. After screening several COFs, the researchers chose one containing bipyridine groups, which anchor the Cu2+ ions, forming a stable composite. Adding copper to the COF broadens the wavelength spectrum of light that the material can absorb, allowing it to utilize more sunlight for heat conversion.
To test the composite, the researchers put it on a simple filter membrane and placed it on simulated wastewater containing penicillin and common organic pollutants. When they shone a UV lamp on the membrane to mimic sunlight, the material reached a temperature over 60 °C, well above the temperature of the surrounding water, speeding up the Cu2+ ion-triggered hydrolysis of the antibiotics, completely degrading the drug within 10 minutes of light exposure.
Tests with real wastewater and other antibiotics will come next, Ma says. Real-world use would entail placing the membrane on the water and focusing sunlight on it, as well as reducing COF cost and finding a suitable membrane support for the catalyst, he says.
The complexity and cloudiness of real wastewater could limit the photocatalytic effect and make implementating the technology a challenge, says Peiying Hong, an environmental engineer at King Abdullah University of Science and Technology. Both the reduced level of light penetration and the membrane fouling due to natural organic matter would need to be addressed for practical use.
Richard Watts, a civil and environmental engineer from Washington State University, agrees. Nonetheless, he says, the research is novel and based on a promising principle. “The system appears to be innovative and might have other applications.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter