Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.

Agriculture
The industrialization of the Haber-Bosch process
Nitrogen fertilizer fuels climate change, pollutes drinking water—and feeds nearly half the planet
by Matt Blois
August 11, 2023
| A version of this story appeared in
Volume 101, Issue 26

Earth is bathed in nitrogen. It makes up about 78% of the atmosphere, and it’s stitched into every nucleotide, every cell, every living thing on the planet. But before humans learned to synthesize ammonia using the Haber-Bosch process, nitrogen was a major constraint on the proliferation of life.


Nearly all nitrogen in the atmosphere comes in the form of N2, an inert molecule unsuitable for the chemistry that supports life. Living things need reactive nitrogen, which requires breaking the powerful triple bond holding dinitrogen’s two atoms together.
For the first few billion years of life on Earth, there were two ways to convert the oceans of inert N2 into life-sustaining reactive nitrogen: the massive, sudden heat of a lightning strike ripping through the atmosphere or microbes sucking in air and using an enzyme to slice dinitrogen’s triple bond.
In the early 20th century, the chemist Fritz Haber created a third way. He reacted N2 with hydrogen under intense heat and pressure in the presence of a catalyst to form ammonia. A few years after Haber patented his discovery, the BASF engineer Carl Bosch helped transform the laboratory experiment into an industrial operation. The technique was labeled the Haber-Bosch process. The International Fertilizer Association reports that it was used to make roughly 150 million metric tons of ammonia in 2021.
Around the time of Haber’s discovery, scientists were warning that natural sources of reactive nitrogen, such as bird guano from Pacific islands or nitrogen salts found in South America’s deserts, would not provide enough fertilizer to feed the world’s growing population and that millions would starve without a new way to produce nitrogen.
Benjamin Houlton, an environmental scientist at Cornell University, argues that the Haber-Bosch process played a critical role in preventing this tragedy by massively increasing agricultural production. “Nitrogen is the key that unlocked the global food system,” he says.
In the 1930s, US farmers grew less than 1,500 kg of corn per hectare, according to data from the US Department of Agriculture. Since the start of this decade, they have produced an average of over 10,000 kg of corn per hectare. A 2008 study in Nature Geoscience estimates that without the Haber-Bosch process, about half the world’s population wouldn’t have enough food.

This influx of nitrogen has caused serious environmental problems. Fertilizer runoff pollutes drinking water and threatens species with extinction. Nitrogen gases released when fertilizer is applied cause air pollution, and the Haber-Bosch process itself is a major contributor to climate change, responsible for about 1% of all human-made carbon dioxide emissions.
Source: Nat. Geosci. 2008, DOI: 10.1038/ngeo325.
Note: Data after 2008 are estimates.
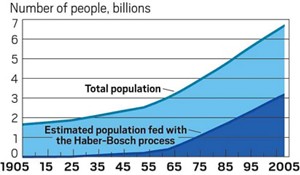
Source: Nat. Geosci. 2008, DOI: 10.1038/ngeo325.
Note: Data after 2008 are estimates.
But Dan Blaustein-Rejto, director of food and agriculture at the Breakthrough Institute, a think tank focused on sustainability, points out that feeding the world without fertilizer would require much more land, which would mean removing forests and other ecosystems that store lots of carbon and reducing the amount of habitat available for wildlife. Without nitrogen fertilizer, agriculture’s environmental footprint could very well be larger, he says. “Despite its poor environmental reputation, nitrogen fertilizer has actually been key to limiting agriculture’s land footprint.”
Houlton agrees that the Haber-Bosch process has delivered enormous benefits but says the costs are starting to add up and shouldn’t be ignored, especially as the world’s appetite for nitrogen continues to rise. “The positives are almost infinite in some ways,” he says. “But those negatives can come back to disrupt society as we know it, and I think it’s happening.”
Policies that limit nitrogen pollution and innovations that make fertilizer use more efficient could help capture the benefits while minimizing environmental damage, Houlton says. And those solutions need to be deployed more quickly.
Fertilizer’s Future
Given the negative environmental impacts of making and using synthetic nitrogen fertilizer, many companies and researchers are looking for alternatives to the Haber-Bosch process. Here are a few options.
WASTE NOT, WANT NOT
One of the most straightforward ways to reduce the negative effects of the Haber-Bosch process is to waste less of the fertilizer used on farms. Much of this fertilizer never makes it into a plant; farmers can increase efficiency by applying fertilizer to crops only when and where it’s needed. A recent study in Nature Food calls this approach the most effective way to address greenhouse gas emissions from nitrogen fertilizer. It estimates that emissions could be reduced by 20% using existing technologies.
ELECTRIFYING AMMONIA
Several companies are developing methods that use renewable electricity to produce ammonia, resulting in lower greenhouse gas emissions than the Haber-Bosch process. The start-up Nitricity zaps the air with plasma reactors powered by renewable energy, creating reactive nitrogen in a process that mimics lightning. Another firm, Nium, says it can produce ammonia at low temperatures and low pressures with renewable energy by using a new type of catalyst. Major fertilizer producers like Yara and CF Industries Holdings are building green ammonia plants, which use renewable energy and rely on water instead of fossil fuels as a source of hydrogen.
MICROBES TO THE RESCUE
Some plants, such as beans, peas, and other legumes, have symbiotic relationships with bacteria that can absorb atmospheric nitrogen and convert it into reactive nitrogen. Growing these crops can increase the amount of nitrogen available in soil. But these microbes don’t associate with many of the world’s major cereal crops, such as corn, rice, and wheat. Several firms, including Pivot Bio and Ginkgo Bioworks, are developing microbes that have been genetically engineered to produce nitrogen for cereal crops. Farmers used Pivot Bio’s product on more than 1.2 million hectares last year.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter