Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Discovery
An antibiotic built for better binding
Cresomycin’s conformationally restricted structure makes a tight fit to bacterial ribosomes and pushes away methyl groups that can confer resistance
by Bethany Halford
February 19, 2024

Cresomycin—a new antibiotic drug candidate designed to bind tightly to bacterial ribosomes—appears to sidestep a common mechanism bacteria use to gain the upper hand against antimicrobial compounds. Cresomycin was able to fight off both gram-positive and gram-negative bacteria, including multidrug-resistant strains of Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa, in both test tubes and in mice.
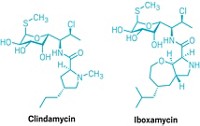
Andrew Myers at Harvard University and Yury Polikanov at the University of Illinois at Chicago led the research effort that produced cresomycin. In 2021, Myers and Polikanov reported iboxamycin, an antibiotic inspired by the drug clindamycin. Iboxamycin preserved one half of clindamycin’s structure but changed the other half in a way that made iboxamycin bind more tightly than clindamycin to their mutual target—the bacterial ribosome.
These antibiotics “bind to the active site of the ribosome and make the ribosome incapable of doing what it’s supposed to do. So it becomes impossible for the ribosome to make a protein,” Polikanov explains.
After studying how iboxamycin binds to the ribosome and studying its conformation computationally, the chemists decided to see what would happen if they modified the previously unaltered half molecule to make a macrocycle that is locked into the most favorable position for binding.
Myers says that several of his students thought conformationally restricting the molecule’s movement would lead to better binding, but the first generation of macrocycles weren’t particularly active. “The idea could easily have been abandoned,” he says in an email. “On careful reconsideration of the problem and the data, my student Kelvin Wu realized that we had targeted the wrong ring size.” That insight led the team to make cresomycin’s 10-membered ring.
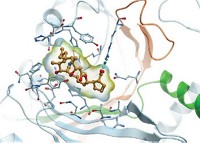
Structural biology studies show that cresomycin binds tightly to several bacterial ribosomes, including drug-resistant ones. “What was most shocking and unexpected is to see how this molecule overcomes resistance,” Polikanov says. The molecule pushes away methyl groups in these drug-resistant ribosomes that prevent other antibiotics from binding. This, Polikanov says, probably occurs because of how well cresomycin engages the ribosome (Science 2024, DOI: 10.1126/science.adk8013).
The study changes the way we think about drug design to combat resistance, says Daniel Wilson, who studies protein synthesis and antimicrobial agents at the University of Hamburg and was not involved in the work. “The drug alterations do not need to be located in the region where the resistance modification lies—it seems that if the affinity is high enough, the drug can simply push the modified nucleotide out of the way to overcome the resistance mechanism,” he says in an email.
Myers recently garnered the Carb-X Award, which will give his lab $1.2 million to move cresomycin and several other compounds through preclinical studies.
Of course, there is an argument that the difficulty making successful antibiotic drugs has more to do with business models than with molecules. When asked about this, Myers says, “Do I worry about the broken business model for antibiotics development? Are you kidding? Every day. That may be the most challenging problem of the lot, and it is not one that I can solve. Synthesizing new antibiotics—in that, I feel confident.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter