Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Alcohols swapped with aromatics
Light-activated reaction offers a general strategy for modifying alcohols
by Bethany Halford
September 7, 2021
| A version of this story appeared in
Volume 99, Issue 33

Alcohols are abundant in the universe of chemicals. But their strong C–O bond makes it tough to alter the motif without modifying it first. Seeking a quick method for transforming alcohols into a wide range of other molecules, chemists developed a cross-coupling reaction that removes alcohols from sp3 carbons and replaces them with aromatic substituents. The reaction could be useful to medicinal chemists because it allows them to tweak complex alcohols to make new molecules that could have improved properties.
Cross-coupling reactions with sp3 substituents often use alkyl bromides, explains Princeton University chemistry professor David W. C. MacMillan, who developed the reaction with Zhe Dong, who was a postdoctoral scholar in MacMillan’s lab and is now a professor at Southern University of Science and Technology. “If you think about the diversity of alkyl bromides that are out there, it’s actually really small,” MacMillan says. “If you do the same analysis for alcohols, you realize it’s huge.”
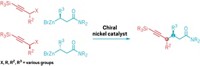
The photoredox reaction uses a commercially available benzoxazolium salt to activate the C–O bond on the alcohol-containing starting compound. A light-activated iridium catalyst then breaks the bond to form a radical on the sp3 carbon atom. At the same time, a nickel catalyst reacts with an aryl halide, which carries the aromatic group that will take the alcohol’s place. This aryl-nickel species traps the carbon radical. Reductive elimination gives the new sp3–sp2 cross-coupled product (Nature 2021, DOI: 10.1038/s41586-021-03920-6).
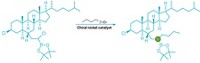
The reaction works with primary, secondary, and tertiary alcohols. “If I see an alcohol, I can now imagine that could be any heteroaromatic, aromatic, five-membered heteroaromatic that I want it to be,” MacMillan says. He and Dong showed that the reaction works on medicinally important molecules by changing the alcohol on a taxol derivative to a heteroaromatic group (shown).
“The era of photoredox methods for building complex organic molecules has truly arrived,” says Jeremy Green, a veteran medicinal chemist and consultant, in an email. He says the work “opens the floodgates” for coupling sp3 carbons to sp2 carbons because it uses accessible alcohol reagents. Green notes that the cost and availability of the iridium catalyst might be a concern.
MacMillan hopes that chemists will find the reaction easy and useful to use. “It’s one thing when you publish a reaction that gets the concept, but it’s another one to actually make it general so that it works so broadly, that people will adopt it,” he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter