Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Carbenes created in a cell
Chemists coax Streptomyces albus into making the carbene precursor azaserine, which reacts with styrene also made biosynthetically
by Bethany Halford
May 3, 2023
| A version of this story appeared in
Volume 101, Issue 15

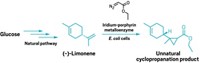
Synthetic chemists have long used carbenes to create cyclopropyl groups and to strategically insert carbon into certain bonds. But these highly reactive intermediates haven’t been available in the realm of synthetic biology—until now. Scientists have created Streptomyces albus cells that can biosynthesize azaserine, a natural product and carbene precursor, and they get it to react with styrene also made by the same cellular system. An engineered P450 enzyme catalyzes the cyclopropanation reaction (shown).
“The whole process end to end is happening in the cell. You basically are adding glucose on one side and getting a cyclopropanated bioactive compound on the other side,” says Aindrila Mukhopadhyay, an expert in microbial processes at Lawrence Berkeley National Laboratory, who was a leader on the project.
The synthetic biology system could help researchers biosynthetically create cyclopropanated versions of natural products, says John F. Hartwig, a chemist at the University of California, Berkeley, who also co-led the project along with colleagues Jay D. Keasling and Douglas S. Clark. The findings suggest synthetic biology could one day also be used to make drugs that contain cyclopropane groups.
Compared with synthetic chemistry, which can use toxic reagents and generate solvent waste, “biology is inherently more sustainable, scalable, and allows more benign processes to be used,” Mukhopadhyay says. The trick is getting this chemistry to take place in a biological system. She creditsBerkeley postdoctoral scholar Jing Huang with orchestrating the complex biochemical processes that build the azaserine and styrene in the cell and also incorporating an engineered P450 enzyme that could catalyze the cyclopropanation reaction (Nature 2023, DOI: 10.1038/s41586-023-06027-2).
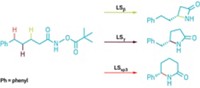
The cyclopropanation reaction had excellent diastereoselectivity, preferably producing one of four possible diastereomers. The researchers also demonstrated that they could use the azaserine to insert a carbene into a carbon-hydrogen bond in phthalan. Next, Hartwig says, the chemists on the team would like to improve the C–H insertion capabilities and to do the cyclopropanation on substrates other than styrene.
Frances H. Arnold, an expert in enzyme evolution at the California Institute of Technology, says in an email that the work “is a tour-de-force integration of natural and ‘new-to-nature’ biocatalysis.” She also says the researchers show “that enzymes can do chemistry that once belonged only to humans—such as alkene cyclopropanation—and in many cases do it better than humans. Such enzymes can greatly expand the scope of chemicals and materials we can build using synthetic biology, as beautifully demonstrated here.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter