Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biocatalysis
Hans Renata
Chemoenzymatic conductor is using enzymes to accomplish exquisitely selective chemical reactions
by Ryan Cross
August 20, 2021
| A version of this story appeared in
Volume 99, Issue 30

Credit: Courtesy of Scott Wiseman; Protein Data Bank ID 3QI8 (Structure)
Hans Renata is a master of chemoenzymatic synthesis, the science—and one might say, art—of using enzymes during key steps in the synthesis of complex molecules.
At 14, Renata left home in Surabaya, Indonesia, to study at a rigorous boarding school in Singapore, which ultimately led him to Columbia University. As an undergraduate, his chemical problem-solving ability attracted the attention of eminent chemists, including the late Gilbert Stork and Ronald Breslow. “They were telling me they had a genius in their midst,” recalls Phil Baran, a chemist at Scripps Research in California.
Advertisement
Baran recruited Renata to Scripps, where as a graduate student he developed new strategies for synthesizing highly hydroxylated steroids. These molecules can be hard to make because the synthesis requires adding the oxygen-containing hydroxyl groups to specific positions on a steroid’s unreactive 17-carbon skeleton. And with so many carbons, it’s hard to get the hydroxyls positioned and oriented in just the right way.
As a challenge, Baran and Renata decided to synthesize one of the most complex steroids they could think of: ouabagenin, a modified natural product with six hydroxyls. After 5 years of work, Renata devised a way to make the steroid in 21 steps, a huge improvement over a previous synthesis of the molecule that required 41 steps.
The achievement earned Renata a
Arnold is a pioneer of using the technique of directed evolution to create enzymes with new functions, and she won the 2018 Nobel Prize in Chemistry for it. As a postdoc in her lab, Renata learned the ropes of how synthetic biology could be used to make enzymes that catalyze reactions not known to occur in nature.
In 2016, Renata began his own lab at Scripps Research in Florida, where he is particularly interested in synthesizing natural products, especially ones that are notoriously hard to make. “The goal of my lab is to simplify how we make these types of molecules by combining chemistry and synthetic biology,” he says.
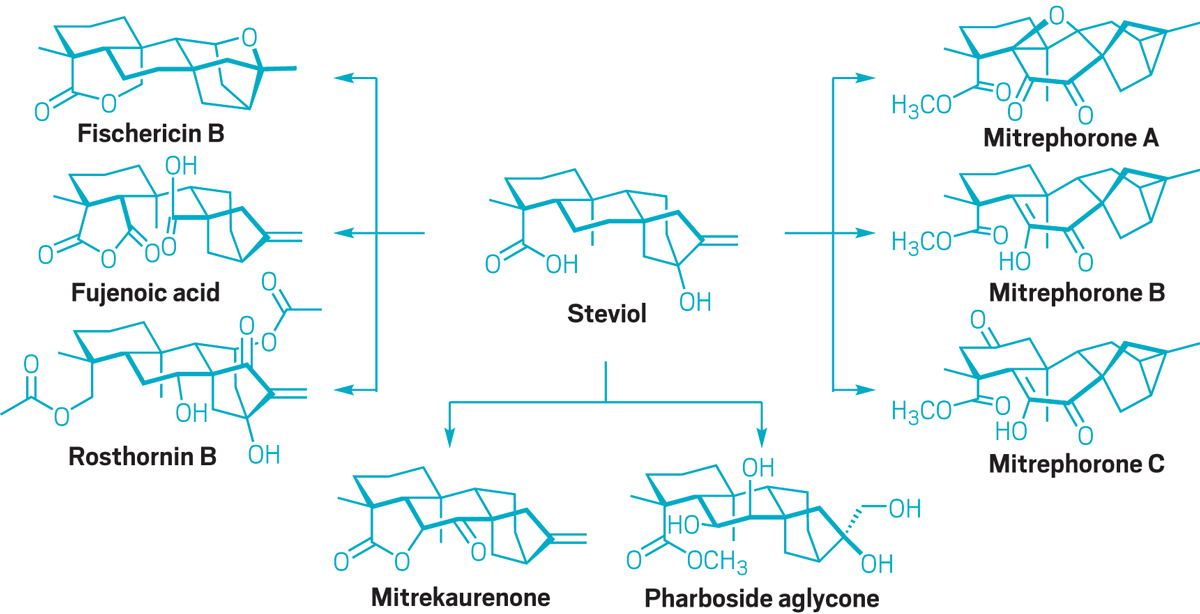
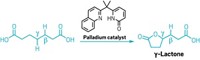
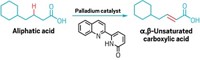
Baran points to two papers published in 2020 that highlight Renata’s creative strategy: one in
Baran calls it “a different type of synthesis going on than anything else out there,” and he thinks Renata’s work will spur more chemists to think about incorporating enzymes in the synthesis of complex, highly oxygenated compounds. Renata has also used his chemoenzymatic approach to make a potential anticancer compound and to develop unnatural amino acids that could be used as the building blocks of peptide or protein therapies.
“He makes molecules that others have a really tough time making,” Arnold says. “People just love his papers,” she adds. “And he just continues to knock out one beautiful demonstration after another.”
One of those admirers is Ingo Hartung, director of medicinal chemistry at Merck KGaA. He says that many synthetic chemists would never touch an enzyme, and he calls Renata a pioneer for merging two disparate fields. “It is like two different planets,” he says. “Enzymes allow you to make completely different chemical connections, which if you are trained as a classical organic chemist, you wouldn’t even think about,” Hartung says. “It’s like playing chess and coming up with a completely new move.”
Vitals
Current affiliation: Scripps Research (Florida)
Age: 36
PhD alma mater: Scripps Research (California)
Hometown: Surabaya, Indonesia
If I were an element, I’d be: Bismuth. “It is extremely stable despite being a heavy element, which mirrors my stoic and reticent nature. As chemical compounds, it exists in +3 and +5 oxidation states, which resonates with the many dualities in my life.”
Role model: Stuart Schreiber. “He was one of the leaders in synthetic organic chemistry in the 1980s and ’90s, then decided to teach himself biology and became one of the pioneers of chemical biology as we know it today.”
Hans Renata is a master of chemoenzymatic synthesis, the science—and one might say, art—of using enzymes during key steps in the synthesis of complex molecules.
At 14, Renata left home in Surabaya, Indonesia, to study at a rigorous boarding school in Singapore, which ultimately led him to Columbia University. As an undergraduate, his chemical problem-solving ability attracted the attention of eminent chemists, including the late Gilbert Stork and Ronald Breslow. “They were telling me they had a genius in their midst,” recalls Phil Baran, a chemist at Scripps Research in California.
Vitals
▸ Current affiliation: Scripps Research (Florida)
▸ Age: 36
▸ PhD alma mater: Scripps Research (California)
▸ Hometown: Surabaya, Indonesia
▸ If I were an element, I’d be: Bismuth. “It is extremely stable despite being a heavy element, which mirrors my stoic and reticent nature. As chemical compounds, it exists in +3 and +5 oxidation states, which resonates with the many dualities in my life.”
▸ Role model: Stuart Schreiber. “He was one of the leaders in synthetic organic chemistry in the 1980s and ’90s, then decided to teach himself biology and became one of the pioneers of chemical biology as we know it today.”
Baran recruited Renata to Scripps, where as a graduate student he developed new strategies for synthesizing highly hydroxylated steroids. These molecules can be hard to make because the synthesis requires adding the oxygen-containing hydroxyl groups to specific positions on a steroid’s unreactive 17-carbon skeleton. And with so many carbons, it’s hard to get the hydroxyls positioned and oriented in just the right way.
As a challenge, Baran and Renata decided to synthesize one of the most complex steroids they could think of: ouabagenin, a modified natural product with six hydroxyls. After 5 years of work, Renata devised a way to make the steroid in 21 steps, a huge improvement over a previous synthesis of the molecule that required 41 steps.
The achievement earned Renata a
Arnold is a pioneer of using the technique of directed evolution to create enzymes with new functions, and she won the 2018 Nobel Prize in Chemistry for it. As a postdoc in her lab, Renata learned the ropes of how synthetic biology could be used to make enzymes that catalyze reactions not known to occur in nature.
In 2016, Renata began his own lab at Scripps Research in Florida, where he is particularly interested in synthesizing natural products, especially ones that are notoriously hard to make. “The goal of my lab is to simplify how we make these types of molecules by combining chemistry and synthetic biology,” he says.

Baran points to two papers published in 2020 that highlight Renata’s creative strategy: one in
Baran calls it “a different type of synthesis going on than anything else out there,” and he thinks Renata’s work will spur more chemists to think about incorporating enzymes in the synthesis of complex, highly oxygenated compounds. Renata has also used his chemoenzymatic approach to make a potential anticancer compound and to develop unnatural amino acids that could be used as the building blocks of peptide or protein therapies.
“He makes molecules that others have a really tough time making,” Arnold says. “People just love his papers,” she adds. “And he just continues to knock out one beautiful demonstration after another.”
One of those admirers is Ingo Hartung, director of medicinal chemistry at Merck KGaA. He says that many synthetic chemists would never touch an enzyme, and he calls Renata a pioneer for merging two disparate fields. “It is like two different planets,” he says. “Enzymes allow you to make completely different chemical connections, which if you are trained as a classical organic chemist, you wouldn’t even think about,” Hartung says. “It’s like playing chess and coming up with a completely new move.”

















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter