Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Fluorination
Radicals break C–F bonds one at a time
Chemists can stop defluorination of CF3 to make mono- and difluorinated compounds
by Leigh Krietsch Boerner
March 4, 2021
| A version of this story appeared in
Volume 99, Issue 8

Scientists want to be able to easily add fluorines to pharmaceuticals and agrochemicals because F-containing groups can help the molecules slip through cell membranes to where they are needed. Researchers now report that they can better control the synthesis of such mono- and difluorinated molecules, making these important classes of compounds more accessible to medicinal and agricultural chemists. (Science, 2021, DOI: 10.1126/science.abg0781).
Trifluoromethyl compounds are often fluorinating agents of choice because they are inexpensive and readily available. However, past attempts to efficiently swap out just one or two of the F atoms to make mono- and difluorinated compounds with other functional groups have typically failed. Once the first C–F bond is broken, the other two get weaker. As a result, when chemists try to switch out one or two F atoms, they tend to remove all three. In the new work, Yi-Feng Wang and coworkers at the University of Science and Technology and Kendall N. Houk at the University of California Los Angeles found a way to hit the defluorination brakes for two key classes of molecules.
The trick to controlling the reaction was identifying the right radical, one that could selectively attack their trifluoroacetamide and trifluoroacetate starting materials, Wang says. The amino-boryl radical preferentially attacks the starting compound instead of mono- or difluorinated sites, Houk says, since the CF3 carbon is very electron deficient. Unlike with other defluorination reactions, once the radical breaks first C–F bond, it is less attracted to the compound, so the reaction stops before all the fluorines are gone.
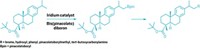
The group used a radical generator and an amino borane to replace C–F bonds with C–H or C–C bonds, adding hydrogen or alkyl groups to make more than 110 mono- and difluorinated acetamides and acetates (example shown). The researchers also modified known drug molecules such as vorinostat, used to treat lymphoma, and norethindrone acetate, a treatment for endometriosis.
Precision defluorination is an important and challenging problem, says Véronique Gouverneur, a fluorine chemist at the University of Oxford. The way that Wang and Houk controlled the reaction was clever and impressive, she says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter