Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
A new heterocycle serves as a piperidine proxy
1-azaspiro[3.3]heptanes subs in for the popular functional group
by Bethany Halford
October 23, 2023
The molecular motif 1-azaspiro[3.3]heptane may look peculiar, with its two 4-membered rings that share a single carbon. But the structure can behave like a mimic of piperidine, a popular heterocycle in many drugs. Until now, however, this molecular motif has been tough to make.
Chemists led by Pavel K. Mykhailiuk at the Kyiv-based pharmaceutical chemical firm Enamine report a synthesis of monosubstituted 1-azaspiro[3.3]heptanes. The route expands the molecular toolbox for medicinal chemists looking to add diverse motifs to their molecules.
2-azaspiro[3.3]heptanes have been popular piperidine mimics, or bioisosteres, since they were first proposed for this purpose in 2010. The motif has appeared in at least 100 research manuscripts, 500 patents, and 7,000 new compounds. But the isomeric 1-azaspiro[3.3]heptane has been rare because there was no modular route to make this motif with just one substituent.
Mykhailiuk and colleagues decided to take up this challenge because of perceived demand from medicinal chemists. “When you know trends well, when you know literature well, when you know what people want, then one of the most interesting parts is to design molecules,” he says.
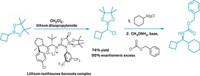
To make monosubstituted 1-azaspiro[3.3]heptanes, the chemists start by doing a thermal [2+2] cycloaddition between an endocyclic alkene and chlorosulfonyl isocyanate to make a spirocyclic β-lactam. Subsequent reduction of the spirocyclic β-lactam with alane-produced a 1-azaspiro[3.3]heptane. The researchers used their synthesis to make an analog of the painkiller bupivacaine (shown), which still retains some of its anesthetic properties in tests with mice (Angew. Chem., Int. Ed. 2023, DOI: 10.1002/anie.202311583).
![The structure of an analog of bupivacaine where the piperidine has been replaced with 1-azaspiro[3.3]heptane The structure of an analog of bupivacaine where the piperidine has been replaced with 1-azaspiro[3.3]heptane](https://s7d1.scene7.com/is/image/CENODS/20231023lnp1-struct-social?$responsive$&wid=700&qlt=90,0&resMode=sharp2)
“Medicinal chemists are always looking for unique bioisosteres to incorporate into their lead molecules, but the great ideas are often met with the sad reality of challenges of incorporating it into a complex scaffold,” Donna Huryn, an expert in medicinal chemistry at the University of Pennsylvania, says in an email. “While azaspiro[3.3]heptanes have been described (albeit sparingly) before, this work demonstrates their efficient synthesis and protocols for further functionalization so that embedding it into a complex molecule isn’t the hurdle that it sometimes is.”
Mykhailiuk tells C&EN that he’s had some interest from medicinal chemists since Enamine started advertising 1-azaspiro[3.3]heptane intermediates for sale a few months ago. “I’ll give it a couple of years before they become common,” he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter