Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Imaging
Photoactivated click-chemistry reaction shows inner lives of cells
Catalyzing a bioorthogonal reaction with light offers a way to reveal molecular interactions in real time
by Alla Katsnelson, special to C&EN
February 2, 2022
| A version of this story appeared in
Volume 100, Issue 5

A fast new light-activated reaction enables a way to visualize rapidly unfolding molecular events and interactions in live cells in real time (J. Am. Chem. Soc. 2022, DOI: 10.1021/jacs.1c10390). The method achieves resolution at the subcellular level and can be used to precisely track how experimental therapeutic compounds engage their targets, the researchers say.
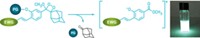
The method uses small-molecule dyes as photocatalysts to turn on a reaction called tetrazine ligation. The dyes catalyze the oxidation of dihydrotetrazine (DHTz), an unreactive precursor, into tetrazine. Tetrazine then participates in a fast click-chemistry reaction with trans-cyclooctene. “It’s a very simple reaction, but of course, we want to do it in a place that’s not simple—inside a living cell or tissue,” says Joseph M. Fox, a chemical biologist at the University of Delaware.
Tetrazine ligation is a bioorthogonal reaction, which means it doesn’t interfere with other cell processes. Harnessing it for fluorescence imaging creates a tool that is much faster and higher resolution than other fluorescent imaging methods and that allows researchers to easily tag proteins at their natural concentrations in the cell, Fox says.
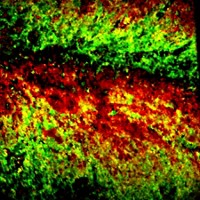
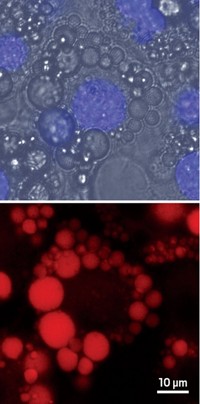
To use the reaction to label cell structures such as mitochondria or the cytoskeleton, Fox and his colleagues expressed a protein—fused to a green fluorescent protein—in cultured cells that homes to an organelle of interest. They then attached DHTz to that fusion protein. The researchers also attached a red dye to trans-cyclooctene and added the resulting molecule to the dish, where it permeated into the cells.

The researchers then added light-activated dyes to serve as photochemical switches for oxidizing the DHTz to tetrazine and to drive the click reaction. DHTz homes to the binding target, and a flash of light from outside the cell at the right wavelength causes the oxidation, with the red trans-cyclooctene molecules moving in and reacting with the newly formed tetrazines, Fox says. Researchers can then visualize the parts of the cell that glow both green and red. And by only shining light on certain parts of the cell, they can control when and where in the cell the reaction gets triggered.
Using the method, the researchers were able to visualize how an inhibitor targets a protein called monoacylglycerol lipase, which naturally occurs in cells at low levels and is being studied as a target in pain-reducing medicine. “The gain of fluorescence is very rapid but not instantaneous,” Fox says. “We can observe the kinetics in real time.”
One dye they used for the oxidation, fluorescein, can absorb a very long wavelength of light—880 nm—and is used with two-photon microscopy, an especially powerful imaging technique. This enabled them to observe structures at the suborganelle level in living cells.
Qing Lin, a bioorganic and medicinal chemist at the University at Buffalo, calls the technique “very exciting.” Tetrazine ligation has been popular among chemical biologists, but most tetrazines tend to be unstable in living cells, he says. “The use of a more stable precursor in the form of dihydrotetrazine coupled with photocatalytic activation to generate highly reactive tetrazines in situ is clever.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter