Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Under pressure, O2 goes to (O2)4
September 18, 2006
| A version of this story appeared in
Volume 84, Issue 38
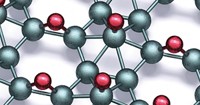
As oxygen is squeezed and solidified under increasing pressure, it passes through a series of six distinct crystal phases, one of which−the ε phase−is marked by a dark red color and the collapse of magnetic behavior. The detailed structure of this phase, which has eluded researchers for 27 years, has now been determined independently by two groups using X-ray diffraction. Their results indicate that in the ε phase, which persists at pressures from 8 to 96 gigapascals, four O2 molecules associate into a rhombohedral O8 unit that is probably held together by weak chemical bonds. These (O2)4 rhombs (shown) are quite different from the long-sought O8 rings that would be an analog of the well-known S8 rings of elemental sulfur. The fact that this rhombohedral tetramer structure hasn't been predicted by theory "presents a challenge to our understanding of dense oxygen," according to a team of physicists from Scotland, Canada, and France, who report the structure in Nature (2006, 443, 201). The other structure elucidation of ε-oxygen was published last month by a Japanese team (Phys. Rev. Lett. 2006, 97, 085503).


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter