Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Made-To-Order Histones
Method for installing methyl marks will shed light on DNA packaging
by Amanda Yarnell
March 12, 2007
| A version of this story appeared in
Volume 85, Issue 11

A new method to chemically synthesize methylated histone proteins will help biochemists figure out how these methyl marks control access to genomic DNA.
Eukaryotic cells must jam their nearly 6 meters of genomic DNA into a nucleus measuring just a few micrometers across. To do so, they wrap their DNA around scaffolding proteins known as histones, which are then further compacted into a higher order structure known as chromatin. But biological machinery such as that responsible for transcribing DNA into RNA must somehow gain access to the packaged DNA. To control such access, cells stud histones' lysine side chains with constellations of methyl, dimethyl, and trimethyl marks.
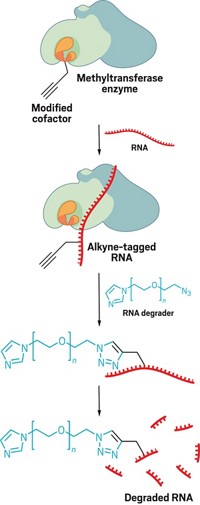
Biologists have struggled to decode the subtle biological directives indicated by different patterns and degrees of lysine methylation on histones. One powerful way to tease out the individual meanings of these marks would be to install methyl groups at will and observe their effects. Toward this end, Kevan M. Shokat and Matthew D. Simon of the University of California, Berkeley, and UC San Francisco, respectively, and coworkers have devised a way to make custom-methylated histones (Cell 2007, 128, 1003).
The route takes a cue from the aminoethylation reaction traditionally used to convert cysteine residues into lysines. The researchers first designed a histone protein containing a single cysteine at the desired site of modification. They then treated the protein with an N-methylated 2-haloethylamine to convert the cysteine into the corresponding N-methylated aminoethylcysteine. Such elaborated cysteine residues behave similarly to their natural methyl lysine counterparts in functional assays, Shokat says.
"This scheme provides a simple and affordable route to large quantities of specifically methylated histones," Shokat says. Such histones will be useful for probing the complex biology of chromatin, he adds.
By opening up an easy route to histones in which the site and degree of methylation can be specified, the new method is poised to "revolutionize the chromatin field," comments chromatin researcher Craig L. Peterson of the University of Massachusetts Medical School.
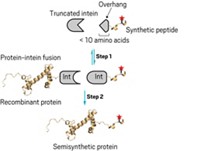
The method is easier to use and more flexible than semisynthetic routes to methylated histones and thus more likely to be adopted by molecular biologists, notes Duke University chemist Dewey McCafferty. "We are hoping to use the method ourselves," Peterson notes.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter