Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Nanoparticles Act Like Atoms
Gold spheres, bestowed with valency, are strung together in polymer-like chain
by Bethany Halford
January 22, 2007
| A version of this story appeared in
Volume 85, Issue 4

Using a little topology and a few thiol ligands, materials scientists have managed to corral a gold nanoparticle's thousands of atoms and make them behave like one divalent atom (Science 2007, 315, 358).
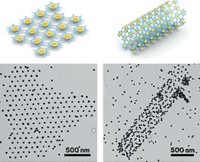
Transformed from a multivalent mass to tidy two-handled building blocks, the nanoparticles can then be hooked together into a tiny string of golden beads. "It's the nanoscale equivalent of a polymer," says Francesco Stellacci, the Massachusetts Institute of Technology professor who spearheaded the research.
To establish an orderly divalent bonding motif in the unruly nanoparticles, Stellacci's group took advantage of what topologists call the "hairy-ball theorem." Basically, the theorem states that if you've got a sphere covered in hair, it's impossible to comb those hairs so they all lie flat. No matter what you do, two hairs at opposite points on the sphere will stand straight up.
Applying the theorem to a gold nanoparticle "sphere" coated with a mixture of two types of thiol ligand "hairs," the researchers reasoned they could swap the thiols at those polar positions with thiol chains bearing a terminal carboxylic acid. "We knew that the exchange reaction would take place orders of magnitude faster there than at any other point on the sphere," Stellacci explains.
With an acid-functionalized chain at each of its poles, the particle selectively bonds with other molecules as though it were a divalent atom. Once they'd done the thiol swap, Stellacci's group simply condensed the divalent particles with 1,6-diaminohexane, creating polymer-like strands up to 20 nanoparticles long.
"Introducing valency into nanostructures through face- or edge-selective functionalization" is a major fundamental advance, comments Northwestern University's Chad A. Mirkin, an expert in controlling nanoscale architecture. The work will help researchers "realize more sophisticated and highly functional architectures," Mirkin adds. "It is analogous to transitioning from atoms to molecules."



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter