Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Enzyme’s Metal Cluster Is Nailed
Definitive solution of the structure of an enzyme in isoprenoid biosynthesis could lead to new antibiotics
by Stuart A. Borman
June 7, 2010
| A version of this story appeared in
Volume 88, Issue 23

A long-standing disagreement over the structure of a key metalloenzyme is over, thanks to efforts by several groups. The enzyme, called IspH, is found in pathogenic microorganisms but not in humans, making it a promising drug target, because medications aimed at it would kill the microorganisms without adversely affecting the people infected by them.
IspH has been an active focus of research by several groups for almost a decade. But difficulties in studying its iron-sulfur cluster and conflicting findings have made its structure and mechanism controversial. Researchers investigating whether the cluster is 3Fe-4S or 4Fe-4S now agree that it is the latter.
Using the recent findings, researchers have identified compounds that inhibit the enzyme and may lead to IspH-targeted medications. And they have proposed that the enzyme’s workings involve organometallic bonding of a type that has only rarely been observed.
The enzyme is present in malaria parasites, pathogenic bacteria, and plants, but not in people or other animals. It catalyzes the reductive dehydroxylation of (E)-1-hydroxy-2-methyl-2-butenyl 4-diphosphate (HMBPP) to two isomeric five-carbon compounds: isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). These two compounds—which are made in many organisms by another enzyme in a different biosynthetic pathway—are the building blocks of all biological isoprenoids, the largest group of natural products, which includes cholesterol, carotenoids, steroid hormones, and vitamins.
The IspH reaction is the final step in the “nonmevalonate” biosynthetic pathway, which was discovered and characterized by microbial bioorganic chemist Michel Rohmer of the University of Strasbourg, in France, and coworkers (Biochem. J. 1993, 295, 517). In bacteria, the pathway leads to isoprenoids involved in processes such as cell wall biosynthesis and quinone formation, which are essential to microbe viability.
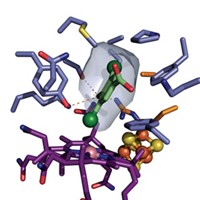
An iron-sulfur cluster serves as an electron-transfer cofactor in the active site of IspH. That cluster’s 4Fe-4S composition took a while to become established.

About two years ago, biochemists Jochen Wiesner and Hassan Jomaa of the University of Giessen, in Germany, and crystallographer Ulrich Ermler of the Max Planck Institute of Biophysics, in Frankfurt, asked metalloprotein and drug discovery specialist Eric Oldfield of the University of Illinois, Urbana-Champaign, to provide computational input on a structural study of IspH. The resulting study found that the cluster contained three Fe atoms (J. Am. Chem. Soc. 2008, 130, 17206).
But in the same study, the researchers proposed a structural model involving a four-Fe cluster and a mechanism that involved binding of HMBPP to one of IspH’s Fe atoms. Earlier electron paramagnetic resonance results by Rohmer and coworkers showed a 4Fe-4S cluster (FEBS Lett. 2003, 541, 115), which made a catalytically active four-Fe structure seem more likely to Wiesner, Jomaa, Ermler, and Oldfield, despite their own study’s crystallographic findings.
The four-Fe model was soon challenged by chemistry professors Adelbert Bacher, Tobias Gräwert, Jörg Eppinger, and Michael Groll of the Technical University of Munich and coworkers, who used crystallographic evidence to propose that IspH’s cluster and mechanism were based on a 3-Fe cluster (Angew. Chem. Int. Ed. 2009, 48, 5756).
At about the same time, two groups working independently—Rohmer’s and that of Boston University isoprenoid pathway specialist Pinghua Liu—used Mössbauer spectroscopy to show that IspH has a 4Fe-4S cluster (J. Am. Chem. Soc. 2009, 131, 9931 and 13184).

This year, the Munich-based team revised its crystallographic model of IspH’s cluster to a four-Fe structure and proposed a corresponding mechanism (Proc. Natl. Acad. Sci. USA 2010, 107, 1077). Their study provides key insights into substrate-enzyme binding interactions and involvement of the fourth Fe in the formation of early and late reaction intermediates, Groll says.
Oldfield and coworkers also confirmed the 4Fe-4S cluster (Proc. Natl. Acad. Sci. USA 2010, 107, 4522). “I think everyone now agrees that a 4Fe-4S cluster is involved,” Oldfield says.
However, controversy still swirls around how the cluster transfers electrons. No fewer than six competing mechanistic proposals have been floated to date. In their PNAS paper, Oldfield and coworkers proposed an organometallic mechanism for IspH, in which HMBPP bonds transiently to one of the cluster’s Fe atoms.
The team has now shown that IspG, IspH’s predecessor in the nonmevalonate pathway, also uses an organometallic mechanism (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.1000264107). They believe other 4Fe-4S-containing enzymes may have organometallic mechanisms as well.
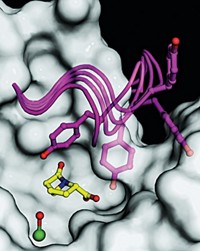
The organometallic mechanistic model has already turned out to be predictive. “It was known that olefins can form π complexes with low-valent Fe and that acetylenes form even stronger bonds,” Oldfield says. Using such clues, his group has now identified acetylene-based IspH inhibitors that are about 1,000 times more potent than any IspH inhibitors developed previously (J. Am. Chem. Soc. 2010, 132, 6719).
The inhibitors have not yet been tested in cells, but a patent is pending. Oldfield and coworkers hope they will lead to effective agents against malaria parasites and other disease-causing bacteria.
Researchers “have marshaled compelling evidence for the 4Fe-4S cluster and its critical role in this enzyme. It’s beautiful bioinorganic chemistry,” comments theoretician J. Andrew McCammon of the University of California, San Diego.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter