Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Environment
Fluorochemicals Go Short
Shorter perfluoroalkyl chain lengths improve environmental profile of versatile stain-, grease-, and water-repelling chemicals
by Stephen K. Ritter
February 1, 2010
| A version of this story appeared in
Volume 88, Issue 5

Nearly all humans, and a large proportion of wildlife, are contaminated with environmentally persistent long-chain perfluoroalkyl compounds. That revelation, around for a decade now, has brought dramatic change to the fluorochemicals industry.
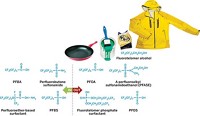
Spurred on by academic researchers and concerns from environmental and consumer advocacy groups, chemical companies have worked with the Environmental Protection Agency to phase out perfluorooctane sulfonate (PFOS) and are in the process of phasing out perfluorooctanoic acid (PFOA). The companies are replacing PFOS, PFOA, and their associated compounds with shorter perfluoroalkyl chain compounds that impart the same functional properties as the longer chain compounds. Although the alternatives are just as persistent, they aren’t as bioaccumulative and appear to have a better toxicity profile—which is still being confirmed by testing—and are thus considered sound replacements.
The chemical inertness of these fluorocarbons and their simultaneous hydrophobic and lipophobic character make them useful for manufacturing a variety of plastics and as surface treatments for industrial and consumer products, explains David W. Boothe, global business manager for DuPont Fluoroproducts. He says people have come to take such products for granted: nonstick cookware, grease-proof fast-food wrappers, stain-resistant carpet, and water-repellent clothing. Less visible are critical applications such as components of fuel hoses for cars, hydraulic fluids for airplanes, insulation for telecommunications wiring, and as surfactants to aid application of pesticides, enhance wetting properties of paints and coatings, and improve flowability of fire-fighting foams.
“The societal benefits of fluoroproducts—boosting gas mileage in cars while cutting air emissions, adding durability to clothing, improving semiconductor and communications cable performance, and increasing fire-fighting speed—help consumers save money and make products safer, last longer, and environmentally friendlier,” Boothe emphasizes.
The downside is that, over time, emissions from the manufacture and use of PFOS and PFOA have meant that they are ubiquitous in the environment, where they have no natural analogs. The compounds are found in places ranging from pristine areas of the Arctic to sludge in municipal wastewater treatment plants. And they’ve infiltrated nearly every corner of the food chain, from herring to humans.
PFOS and PFOA are known to exhibit toxicity in lab animals, but so far their toxicity to people is uncertain, says Toni Krasnic, coordinator for perfluorochemicals in EPA’s Office of Pollution Prevention & Toxics. EPA has yet to make a full judgment of whether these compounds pose an unreasonable risk to the public, Krasnic says. One holdup is that to effectively deal with PFOS and PFOA, the research community has to scrutinize the compounds’ associated precursors and derivatives, too. “It makes the whole risk-management issue more complicated,” Krasnic says.
Scientists studying the fluorochemicals agree that, given the persistence, long-term exposure to PFOS and PFOA and their associated compounds can reasonably be anticipated to result in adverse health effects. But at the same time, the fluorochemicals are too useful to society and are too large of a market for chemical companies to abandon. Those realizations prompted EPA and chemical firms to start working toward finding sustainable solutions.
The PFOS and PFOA families of compounds have been produced since the late 1940s. PFOS is a degradation product of perfluorooctane sulfonamide derivatives, including perfluoroalkyl sulfonamidoethanols (PFASEs), that were originally the key components used to make 3M’s Scotchgard brand of stain-protection products.
PFOA’s ammonium salt and, to a lesser extent, its longer perfluorononanoic homolog have been used in small amounts as surfactants to help solubilize fluorinated monomers in the industrial emulsion polymerization of tetrafluoroethylene to produce DuPont’s Teflon and other fluoropolymers. PFOA and other perfluoroalkyl carboxylic acids (PFCAs) are also inadvertent degradation products of long-chain fluorotelomer alcohols (FTOHs), a set of oligomeric compounds built up from tetrafluoroethylene by a free-radical telomerization process. Chemical companies use PFASEs and FTOHs to add fluorinated pendant groups to polymers and surfactants that are then used to coat metals, paper, carpet, and fabrics.
Fluorinated compounds were first noted in human blood in the late 1960s, and by 2000, PFOS and PFOA were specifically identified and discovered to be virtually anywhere scientists checked. PFOS currently is found at higher concentrations in blood than PFOA, about 20 ppb versus 4 ppb on average in the U.S. Chemical production workers tend to have levels about 100 times higher, according to EPA data.
Animal studies indicate that when the chemicals are at moderate to high levels—mostly above 1,000 ppb—they cause developmental problems, liver toxicity, immune system problems, and benign tumors. In some people, higher levels of PFOS and PFOA correlate with higher cholesterol levels and a higher incidence of thyroid disease, as noted in studies based on data from the National Health & Nutrition Examination Survey conducted by the Centers for Disease Control & Prevention. But other studies by academics, EPA and other federal agencies, and the companies themselves indicate that the chemicals so far are not firmly associated with any increased risk for cancer or other adverse health effects in people.

Nevertheless, when it became clear that PFOS was accumulating in people and the environment, 3M took a proactive step and, in cooperation with EPA, voluntarily began phasing out production of PFOS, PFOA, and PFOS-related products in May 2000. It was a daring move for 3M, the leading global producer and only U.S. maker of the PFOS family of compounds. The company decided to take a chance on reworking its product line to sustain its $300 million business.
In 2003, 3M unveiled a new version of Scotchgard, reformulated with chemistry based on perfluorobutane sulfonamide and its related alcohol in place of PFOS chemicals. The ultimate degradation product, perfluorobutane sulfonate, has a much shorter half-life in people: about one month versus 5.4 years for PFOS and 3.8 years for PFOA.
EPA went on to use provisions of the Toxic Substances Control Act to limit future U.S. manufacturing and import of PFOS and its related chemicals, Krasnic notes. As a follow-up, EPA expanded its investigation of PFOA.
As a result, in January 2006, EPA and the eight major companies that make or use PFOA launched the 2010/2015 PFOA Stewardship Program to formally put a stop to environmental release of PFOA and its related compounds. The companies—Arkema, Asahi, Ciba, Clariant, Daikin, DuPont, 3M/Dyneon, and Solvay Solexis—committed to reducing global facility emissions and product content of PFOA and its associated compounds by 95% relative to 2000 levels by the end of this year. The companies also committed to working toward fully eliminating PFOA emissions and product content by 2015, leading the firms to start switching over to shorter chain replacements.
The impact of the 3M phaseout and the PFOA stewardship program is already evident, Krasnic says. PFOS levels in U.S. adult blood donors dropped by 60% and PFOA levels by 25% from 2000 to 2006, according to a study by 3M scientists (Environ. Sci. Technol. 2008, 42, 4989).
“On the one hand, this study shows that what we are doing is effective,” Krasnic says. “On the other hand, we see a trend that as we phase out these chemicals in the U.S., they are being picked up in other parts of the world, such as China.” If manufactured goods containing these chemicals are imported, there will still be a source of exposure in the U.S., which could undermine the transition to the short-chain alternatives, he points out.
Industry efforts to phase out the problematic long-chain fluorochemicals have stimulated an evolution in the scientific understanding of PFOS and PFOA. These developments have required the efforts of many academic scientists around the world—in some cases, working side by side with EPA and company scientists—to paint a clearer picture of how perfluorochemicals have infiltrated the environment and our bodies.
In 2006, a landmark study by Konstantinos Prevedouros and Ian T. Cousins of Stockholm University, in collaboration with DuPont’s Robert C. Buck and Stephen H. Korzeniowski, provided the first detailed account of the global production history, direct and indirect emissions, and fate of PFOA and its homologs (Environ. Sci. Technol. 2006, 40, 32). Prevedouros and coworkers estimated the global historical emissions of PFOA from 1951 to 2004 for direct manufacture and use, plus indirect sources such as residuals in finished products.

They determined that up to 6,900 metric tons of PFOA, or around 10% of total production, was directly emitted from chemical plants. Most of the material was released in process wastewater prior to 2000, before companies significantly tightened their emissions controls. An additional 350 metric tons was indirectly emitted. The team also determined that ocean surface waters contain a majority of the compounds and that ocean transport is responsible for most of the distribution around the globe.
But the ocean transport mechanism can’t fully explain how PFCAs have ended up on land in remote locations away from water. Scott A. Mabury of the University of Toronto, a leading PFOS/PFOA researcher, reasoned that there must be an atmospheric transport component in addition to the oceanic transport mechanism described by the Stockholm researchers.
As laid out in a series of papers over the past decade, Mabury and coworkers studied the physical properties of PFOA, FTOHs, and related compounds independently of available chemical company data and showed that the compounds can be found in the atmosphere and in indoor air. Mabury’s group, in collaboration with Derek C. G. Muir of Environment Canada, also conducted monitoring studies to show that PFCAs with chain lengths up to C15 are in the Great Lakes and in the water and snow across remote regions of the Canadian Arctic. In these locations, the fluorochemicals exist at concentrations that match the researchers’ model predictions for atmospheric transport of small amounts of the compounds.
In other modeling studies, James M. Armitage in Cousins’ group at Stockholm evaluated the global transport and projected future trends in PFCA distribution in the environment out to 2050 (Environ. Sci. Technol. 2006, 40, 6969, and 2009, 43, 5830). The team’s key observation is that although PFCA concentrations near chemical plants should decline as producers phase out the chemicals, concentrations in the Arctic marine environment will continue to increase until about 2030, as PFCAs are globally distributed. The group has also explored the transport and fate of PFOS, but that effort has been hampered by lack of complete data on global emissions (Environ. Sci. Technol. 2009, 43, 9274).
Robin Vestergren in Cousins’ group has additionally tracked pathways of exposure to PFCAs, finding that human exposure is fundamentally different from that of wildlife (Environ. Sci. Technol. 2009, 43, 5565). For example, PFOA is more prevalent in people, but the longer perfluorononanoic acid tends to be more common in animals, Vestergren notes.

Food is now the likely dominant pathway of PFOA exposure for most people, Vestergren says. However, for those living in areas near chemical plants that make or use the chemicals, drinking water is likely the more dominant source. Inhalation or other exposure to residual compounds such as FTOHs in consumer materials followed by metabolism is another source, he notes. So even with the PFOA phaseout slated to take place by 2015, “people and wildlife will still continue to receive a fairly constant low-dose exposure far into the future,” Vestergren adds.
Because PFOS and PFOA are the ultimate degradation products for the perfluoroalkyl compounds, they might be the least troublesome of the fluorochemicals in the environment, Mabury believes. His group has been piecing together the chemistry of the sulfonamido and fluorotelomer alcohols in the global environment. Taking into account that PFOS has largely been phased out globally, Mabury has focused more on FTOHs, although most of his observations are relevant to PFASEs as well, he says.
Advertisement
In smog-chamber studies carried out with atmospheric chemist Timothy J. Wallington of Ford Motor Co., Mabury and his colleagues unraveled a degradation pathway between FTOHs and the corresponding PFCAs. They determined that for most of a sample tested, the long perfluoroalkyl chain “unzips” to eventually form carbon dioxide and fluoride ion. But in 1–10% of the sample, the chains remain intact and react with chlorine and hydroxyl radicals, leading to a photochemical chain of events that convert FTOHs to aldehyde and then other intermediates. Ultimately, PFOA and other PFCAs are produced.
Mabury’s research led to a couple of key findings about the perfluoroalkyl chemicals. “The acids bioaccumulate only if there are at least seven perfluorinated carbons in the chain,” Mabury says. “And for each additional carbon, the rate of bioaccumulation goes up by a factor of seven.” Mabury attributes this phenomenon to the hydrophobicity of the compounds, which have a tipping point between six and seven fluorinated carbons. That property is the basis for the C6 and shorter chain replacements, he notes.
In one of the Mabury group’s latest discoveries, Jessica C. D’eon found that fluoroalkyl phosphoric acid diesters used as surfactants on grease-proof food wrappers and pizza box liners occur in high concentrations in human blood bank serum (Environ. Sci. Technol. 2009, 43, 4589). This study marks the first time that a commercial product, rather than PFOS or a PFCA, has been found in human blood, Mabury notes. “No one thought that these chemicals came off the paper,” he says. “It seems to confirm that the fluorosurfactants, not just residual PFASEs or FTOHs, can be metabolized and be a significant source of PFOS and PFOA in humans.”
All that research has finally led Mabury to what he thinks is the key point of the PFOS/PFOA story. Some of the intermediates in the FTOH-to-PFCA pathway are 10,000 times more toxic than PFOA to Daphnia, a freshwater planktonic crustacean used as a “canary” for studying toxicity of chemicals in aqueous systems, Mabury says.
One unique aspect of the PFOS and PFOA compounds is that they are not lipophilic like other persistent organic pollutants. So instead of binding to lipids and being stored away in fat, the electrophilic intermediates might form covalent adducts with proteins and nucleic acids, Mabury says. That has several implications in the hunt for more sustainable replacements, he notes.

The new shorter chain compounds have less steric hindrance than the longer chain analogs and thus a higher potential to interact with biomolecules, Mabury believes. “It may be that the longer chain compounds are less toxic because of steric hindrance,” he says. “But it also is possible that if the shorter chain compounds are more reactive, then their half-lives are so short that their toxicity is less important. That is something we still need to find out.”
In addition, an important difference between the PFOS and PFOA groups of chemicals is that the sulfonamide group in PFASEs acts like a wall between the reactive functional group and the nonreactive perfluoroalkyl chain, limiting further reactivity. FTOHs don’t have that wall, Mabury points out, meaning they are more likely to form electrophilic intermediates and potentially be more toxic.
Either way, Mabury is convinced that all perfluoroalkyl compounds with reactive end functional groups—regardless of chain length—will ultimately end up as stable perfluoro acids in the environment. He agrees that removing residuals such as FTOHs and going with the shorter chains is a start toward reducing exposure. But to fully resolve the perfluorochemical problem, chemical makers will need to alter the basic chemistry so the molecules can degrade to innocuous compounds in the environment, Mabury suggests.
Currently, there are no U.S. standards for PFOS or PFOA in food or drinking water. But now that PFOS and PFOA are becoming better understood, federal agencies are edging toward stronger regulatory control (see page 24). Several states have already set their own drinking water guidance in the range of 0.04 to 2.00 ppb. In January 2009, EPA announced nonbinding provisional health advisories for drinking water of 0.2 ppb for PFOS and 0.4 ppb for PFOA.

And DuPont and 3M joined forces to fund a study led by former EPA toxicologist Robert G. Tardiff of the Sapphire Group consulting firm to determine a “drinking water equivalent level” (Food Chem. Toxicol. 2009, 47, 2557). The study concluded that a safe level of lifetime exposure to PFOA in drinking water is up to 6.5 ppb; average levels of PFOA in drinking water are below 1 ppb, with the exception of water found near some industrial sites, where the average level is 3.5 ppb, according to the study.
In May of last year, PFOS and its associated chemicals were among nine new sets of chemicals added to the original “dirty dozen” list of persistent and bioaccumulative chemicals under the Stockholm Convention on Persistent Organic Pollutants (C&EN, May 18, 2009, page 9). And last month, EPA established the first “chemical action plans” for certain chemicals, including PFOS and PFOA, which could extend the reach of the PFOA stewardship program to include a ban on some chemicals (C&EN, Jan. 11, page 9).
“We now have a lot of the pieces to understand PFOS and PFOA—we know about exposures from water, air, and food—but we still are not certain how important each of them is,” EPA’s Krasnic says. EPA is working to determine if revised drinking water health advisories are needed for PFOS and PFOA. That assessment, scheduled to be completed by the end of this year, “will in part drive whatever additional action we will need to take,” he adds.
Like other companies in the stewardship program, DuPont has been busy introducing new technologies as it eliminates PFOA and long-chain FTOHs. For example, the company’s new Echelon technology removes 99% of residual PFOA from metal, fabrics, architectural materials, and electronics coated with fluorotelomer polymers. The company also developed short-chain PFOA replacement technology for fluoropolymer production and has used it in global manufacturing facilities to make product test materials for its customers and for regulatory approval.
“Our replacement technologies aren’t just a drop-in solution,” Boothe says. “We are talking about hundreds to thousands of variations of these products that each have to be qualified with our customers and their customers all the way down the value chain in order for them to be viable. We took a risk in committing significant resources to address this issue, and that risk paid off—this was a big transformation.”
EPA is taking a more active role in working with companies as they develop the short-chain replacements, Krasnic says. “To date, we have seen more than 100 chemical replacements for the PFOS and PFOA chemicals,” he notes.
As the new chemicals are evaluated and go on the market, they are accompanied by a binding agreement that puts limits on the production and use, Krasnic adds. If data in the future show that there is a concern for these chemicals, the agreements serve as a mechanism for dealing with them without having to take regulatory action.
Looking further down the road at possible nonfluorinated alternatives or compounds that are fluorinated differently, “we are seeing some of those as well,” Krasnic notes. “But so far that is confidential business information that EPA can’t disclose. A lot of companies have made it clear that the shorter chain fluorochemicals are not the final PFOS or PFOA replacements but are intermediate chemicals until better alternatives can be developed.
“We realize we have to be really careful in how we deal with any new compounds that come in because the problems with PFOS and PFOA were a surprise to us in 2000,” Krasnic adds. “We don’t want to be surprised again.”







Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter