Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Radical Ways To Trifluoromethylate
Medicinal Chemistry: Mild methods provide access to new pharmaceuticals and agrochemicals
by Bethany Halford
December 12, 2011
| A version of this story appeared in
Volume 89, Issue 50

Chemists preparing analogs of pharmaceuticals and agrochemicals now have two simple tools for blocking metabolic hot spots on aromatic rings and heteroaromatic compounds, thanks to two mild trifluoromethylation methods. The reactions provide quick access to compounds that may have improved metabolic stability and may have more favorable electrostatic interactions with their targets compared with their parent compounds.
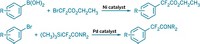
Both reactions trifluoromethylate C–H bonds directly, instead of requiring a prefunctionalized arene. The most recently reported reaction, developed by Princeton University chemists David W. C. MacMillan and David A. Nagib, uses a mild method to generate a trifluoromethyl radical using trifluoromethanesulfonyl chloride, a commercially available ruthenium photocatalyst, and a lightbulb (Nature, DOI: 10.1038/nature10647).
A comparably mild method that uses sodium trifluoromethanesulfinate (Langlois reagent) and tert-butylhydroperoxide to create CF3 radicals, as well as their reaction with heteroaromatics, was reported earlier this year by Phil S. Baran and coworkers at Scripps Research Institute (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.1109059108). In both cases, the electron-deficient CF3 radical readily attacks electron-deficient aromatics, something that both Baran and MacMillan say they found quite surprising.
Chemists have known how to generate CF3 radicals for decades, but the conditions under which they do so tend to be too harsh for functionally complex pharmaceuticals and agrochemicals. The transformations developed by both the Princeton and Scripps teams take place at room temperature and use readily available reagents. Both Baran and MacMillan tell C&EN that their trifluoromethylation reactions have already been adopted by medicinal chemists at several pharmaceutical firms.
The researchers also show the utility of their trifluoromethylation reactions by applying them to existing drug molecules. In one example, Baran’s team prepared two trifluoromethylated analogs of varenicline, sold commercially as Chantix to fight nicotine addiction. Among myriad examples, MacMillan and Nagib created two trifluoromethylated versions of the painkiller ibuprofen and three trifluoromethylated analogs of cholesterol-lowering atorvastatin (Lipitor).
The reactions have modest selectivity, the researchers say, tending to add a CF3 group at the most electron-rich carbon in a substrate. Because these are the sites that tend to be attacked in metabolic processes, MacMillan notes, blocking them with a CF3 group could lead to longer-lived species.
“These breakthroughs open the door to the preparation of a diverse range of molecules that would previously have been difficult to access,” write MIT chemists Andrew T. Parsons and Stephen L. Buchwald in a commentary on the mild trifluoromethylations. “The ability of these streamlined approaches to perform late-stage trifluoromethylations on structurally complex molecules will undoubtedly have an impact on the work of research chemists in the pharmaceutical, agrochemical, and other industries.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter